INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a life-saving therapy that can be used in patients with severe refractory respiratory or cardiac failure. Despite its effectiveness, this rescue therapy is indicated in selected patients whose long-term survival with good neurologic outcome is expected, because it requires considerable financial and human resources. Cardiac arrest patients who remain comatose after resuscitation frequently experience hemodynamic instability, such as cardiogenic shock or recurrent arrest [1,2]. These catastrophic events occur mostly within 24 hours after restoration of spontaneous circulation (ROSC) from cardiac arrest and frequently lead to early death [3]. However, during this period, predicting a patientŌĆÖs neurologic outcome is difficult as the patient requires targeted temperature management (TTM), and sedation and/or paralysis may complicate the clinical picture [4]. As such, the eventual neurologic outcome for a cardiac arrest patient is often uncertain during these critical hours, thus making decisions on ECMO placement more challenging. Amplitude-integrated electroencephalography (aEEG) is an electrophysiologic monitoring tool that uses filtered and compressed electroencephalography from single- or two-channel recordings that has been increasingly used to monitor brain activity in patients with acute neurologic injury. A recent study has suggested that aEEG could be used to predict a good neurologic outcome in the early post-resuscitation period and reported that a continuous normal voltage (CNV) pattern, or the conversion to a CNV pattern within 24 hours post-ROSC, accurately predicted a good neurologic outcome [5].
In this report, we describe our experience of using aEEG to help decide whether to place ECMO for comatose cardiac arrest patients whose eventual neurologic recovery was uncertain at the time of ECMO placement. These cases suggest that aEEG can assist in ECMO decision-making for comatose cardiac arrest patients, particularly during the early post-resuscitation period.
CASE REPORT
Case 1
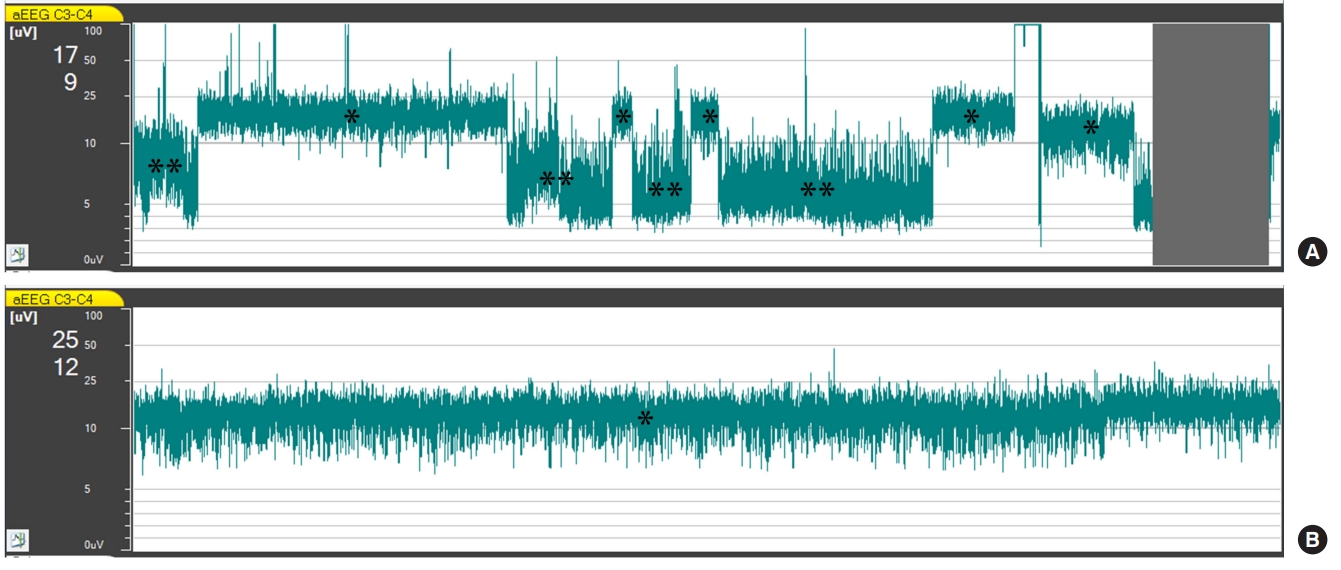
A 38-year-old man was referred from a local hospital after resuscitation from 30 minutes of ventricular fibrillation out-of-hospital cardiac arrest. He was comatose with a heart rate of 102 beats/min and systolic blood pressure of 68 mmHg on arrival. He experienced recurrent arrest 65 minutes after ROSC and was successfully resuscitated again after 20 minutes of cardiopulmonary resuscitation. His post-ROSC electrocardiogram showed prominent ST segment elevations in the anterior precordial leads. He underwent successful percutaneous coronary intervention (PCI) for an obstructive lesion in the left anterior descending artery 40 minutes after ROSC. TTM was instituted and aEEG monitoring was started 1 hour after ROSC. Despite the coronary intervention and the high-dose vasopressor therapy, hypotension persisted. Discontinuous normal voltage (DNV) and CNV patterns appeared alternately, according to his blood pressure measurements, that is, a CNV pattern appeared when his blood pressure increased, and vice versa (Fig. 1). Veno-arterial ECMO was initiated 5 hours after ROSC, and thereafter, aEEG consistently showed a CNV pattern. He regained consciousness on the fourth hospital day. His cardiac function recovered gradually, and he was successfully weaned off ECMO support on the 14th hospital day. However, he developed pneumonia complicated with septic shock on the 23rd hospital day. He died of septic shock on the 36th hospital day.
Case 2
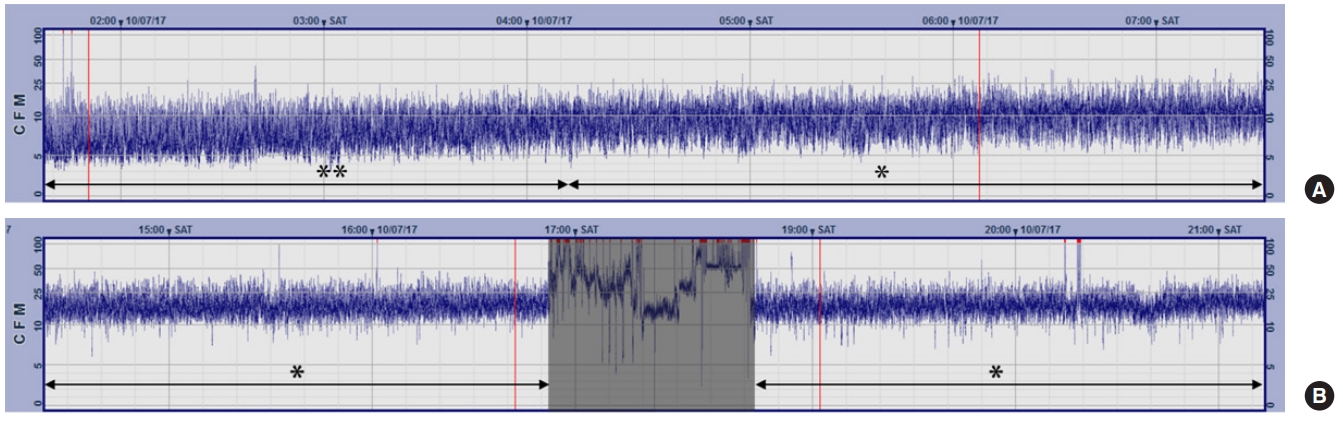
A 48-year-old man was referred from a local hospital after achieving ROSC from 20 minutes of ventricular fibrillation out-of-hospital cardiac arrest. He had a history of prior PCI for non-ST segment elevation myocardial infarction 6 years previously. On admission, he was comatose with a heart rate of 110 beats/min and systolic blood pressure of 100 mmHg. His electrocardiogram on admission revealed ST segment elevations in leads II, III, and aVF. A coronary angiography was performed 75 minutes after ROSC and revealed in-stent restenosis of the right coronary artery. However, the PCI attempts were unsuccessful. We had planned to proceed with a coronary artery bypass graft if the patient regained consciousness after TTM. TTM and aEEG monitoring were initiated 2 hours after ROSC. The initial aEEG showed a DNV pattern, but the DNV converted substantially into a CNV approximately 9 hours after ROSC (Fig. 2). At 22 hours after ROSC, ventricular fibrillation cardiac arrest occurred and remained despite 30 minutes of cardiopulmonary resuscitation. We decided to institute ECMO, and veno-arterial ECMO support was started 1 hour after the onset of the recurrent arrest. He regained full consciousness on the fourth hospital day. A coronary artery bypass graft was performed on the fifth hospital day, and the patient was successfully weaned off ECMO support on the 10th hospital day. He was discharged home without any neurologic sequelae on the 22nd hospital day.
DISCUSSION
Patients who have been resuscitated from cardiac arrest are prone to hemodynamic instability related to acute myocardial dysfunction during the early post-resuscitation period [1,2]. In a study that evaluated the hemodynamic data of 165 cardiac arrest patients during the first 72 hours after arrest, hemodynamic instability, characterized as a low cardiac index, occurred in 90 patients (54.5%) at a median time of 6.8 hours (range, 4.3 to 7.3 hours) [6]. Studies indicate that acute myocardial dysfunction occurring after arrest is reversible in most cases [2,6]. Therefore, ECMO can be employed to salvage cardiac arrest patients with acute myocardial dysfunction unresponsive to vasoactive drugs. Recurrent cardiac arrest occurs early in the course of postarrest care in 24% to 38% of cardiac arrest survivors [1,7] and has been reported to be associated with a decreased likelihood of survival to discharge [1]. Although the use of ECMO in comatose cardiac arrest survivors has not been systematically studied, there appears no reason to not place ECMO in these patients, as long as survival with a good neurologic outcome is likely.
Current resuscitation guidelines recommend a multimodal approach integrating clinical examination findings, electrophysiologic studies, imaging studies, and blood markers to predict outcomes of comatose cardiac arrest survivors [4]. The guidelines also recommend that the prognostication of comatose cardiac arrest survivors should be made later than 72 hours after arrest because TTM itself, as well as sedation and/or paralysis, can confound the accuracies of these modalities [4]. In the cases presented in this report, hemodynamic instability and recurrent cardiac arrest occurred earlier than 24 hours after arrest. Therefore, the results of the prognostication tests, including the somatosensory evoked potential and blood marker neuron-specific enolase tests, were unavailable at the time of decision-making for ECMO. Given that both patients had suffered a considerable duration of ischemia, it was difficult to expect a meaningful neurologic recovery. Nonetheless, the presence of a normal trace on the aEEG at the time of decision-making led us to proceed with the ECMO, and both patients eventually regained full consciousness. Multiple studies have reported that the presence of a normal aEEG trace within 24 hours is a predictor of good neurological outcome after cardiac arrest [5,8]. In a prospective study that included 130 comatose cardiac arrest survivors, Oh et al. [5] reported that the presence of a normal trace within 24 hours predicted a good neurological outcome with a positive predictive value of 88.1%.
Given its easy applicability and interpretability, aEEG is a promising real-time brain monitoring tool that can be used in the management of comatose cardiac arrest patients. Our cases, together with the previous studies, suggest that aEEG can help clinicians in their decision-making processes for advanced treatment such as ECMO in comatose cardiac arrest patients.