AbstractObjectiveTo investigate differences in the effect of intravenous (IV) thrombolysis regarding the mismatch of diffusion-weighted imagingŌĆōfluid-attenuated inversion recovery (DWI-FLAIR) among acute ischemic stroke patients who visited the emergency department (ED) within 3 hours from the onset of symptoms.
MethodsAmong ED patients presenting with an acute ischemic stroke between January 2011 and May 2013 at a tertiary hospital, those who underwent magnetic resonance imaging before IV thrombolytic therapy were included in this retrospective study. Patients were divided into DWI-FLAIR mismatch and match groups. National Institutes of Health Stroke Scale (NIHSS) scores obtained initially, 24 hours after thrombolytic therapy, and on discharge, and early neurologic improvement (ENI) and major neurologic improvement (MNI) were compared.
ResultsDuring the study period, 50 of the 213 acute ischemic stroke patients who presented to the ED were included. The DWI-FLAIR mismatch group showed a statistically significantly greater reduction in NIHSS both at 24 hours after thrombolytic therapy and upon discharge than did the match group (5.5 vs. 1.2, P<0.001; 6.0 vs. 2.3, P<0.01, respectively). Moreover, ENI and MNI were significantly greater for the DWI-FLAIR mismatch group than for the match group (27/36 vs. 2/14, P<0.001; 12/36 vs. 0/14, P=0.012, respectively).
INTRODUCTIONIntravenous (IV) thrombolytic therapy is an established method for treating acute ischemic stroke patients within 3 hours of symptom onset, and recently, the therapeutic time window was extended to 4.5 hours [1-3]. In order to initiate IV thrombolytic therapy, it is critical to know the exact time of symptom onset. However, it is often difficult to obtain the exact time of symptom onset if patients have neurologic deficits such as dysarthria, aphasia, or decreased mental status. Moreover, approximately 25% of ischemic strokes occur during sleep, and patients are often unable to recall the exact time of symptom onset [4-6]. Therefore, the precise time from symptom onset to hospital arrival cannot be documented for the majority of cerebral ischemic stroke patients. Because current guidelines suggest that IV thrombolytic therapy should be performed on the basis of the the last confirmed time of functioning without a neurologic deficit, this requirement may overly restrict use of thrombolytic agents for potential candidates for IV thrombolysis. To help resolve this problem, some investigators have studied whether a mismatch between diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) (DWI-FLAIR mismatch) can be used to select patients who present without documentation of the time of symptom onset but may be eligible for IV thrombolytic therapy [7-15]. There are few studies on the effectiveness of IV thrombolytic therapy between acute ischemic stroke patients with and without DWI-FLAIR mismatching. We hypothesized that acute ischemic stroke patients with a DWI-FLAIR mismatch may better respond to IV thrombolytic therapy than those who do not have a DWI-FLAIR mismatch.
We performed this study to investigate whether there were differences in the effect of IV thrombolytic therapy in the setting of DWI-FLAIR mismatch among acute ischemic stroke patients who visited the emergency department (ED) within 3 hours from the onset of symptoms.
METHODSThis retrospective study was conducted at an ED from January 2011 to May 2013. The study hospital, a tertiary hospital located in Seoul, Korea, has 710 beds. Approximately 37,000 patients visit the ED annually.
We reviewed the medical records of the acute ischemic stroke patients who visited the ED during the study period. We included acute ischemic stroke patients who visited the ED within 3 hours of symptom onset and received IV thrombolytic therapy after MRI examination; patients who received IV thrombolytic therapy before MRI examination or were treated with additional interventions, such as percutaneous thrombectomy, were excluded. Patients with insufficient medical record documentation also were excluded.
The time of symptom onset was determined on the basis of the most recent time of baseline neurologic functioning. To protect personal information, patient name, hospital number, date of birth, and social security number were deleted after assigning each case a serial number. This study was conducted in accordance with the principles of the Declaration of Helsinki [16,17].
Baseline characteristics and factors affecting treatment included age, gender, previous history of disease (for example, stroke, hypertension, diabetes mellitus, hyperlipidemia), current smoking status, current use of antiplatelet agent medications, vital signs (initial heart rate, systolic blood pressure, diastolic blood pressure, as well as those documented prior to IV thrombolytic therapy), administration of antihypertensive drugs before IV thrombolytic therapy, and initial National Institutes of Health Stroke Scale (NIHSS) score. We also reviewed the times between symptom onset and MRI examination, symptom onset and initiation of drug therapy, and time of MRI and initiation of drug therapy.
MRI examination was performed using a 1.5-T scanner (Intera; Philips Medical Systems, Best, The Netherlands). Axial T1-weighted, axial T2-weighted, axial DWI, axial FLAIR, and apparent diffusion coefficient were included as part of the routine MRI protocol at our hospital for acute ischemic stroke patients.
We defined DWI-FLAIR mismatch as a new hyperintense lesion on DWI without findings of hyperintense signal change on FLAIR. Three emergency medical physicians independently determined the presence of DWI-FLAIR mismatching. Each of the three emergency physicians had 2 years of experience in interpreting diffusion-weighted MRI for diagnosing acute ischemic stroke. The ED physicians consulted with a radiologist before the start of study, but the radiologist was not directly involved in this study. The order of cases was randomized, and three identification number lists with a different order of cases were distributed to the three ED physician interpreters. The DWI images were first evaluated, followed by a review of the FLAIR images. Focal signal changes in brain parenchyma were assessed, excluding intra-arterial signal changes. When there was a difference in MRI assessment among interpreters, the final results were determined when at least 2 observers arrived at the same MRI interpretation. On the basis of these results, the patients were divided into two groups: DWI-FLAIR mismatch and DWI-FLAIR match. We determined the location of cerebral ischemic stroke according to the cerebral vascular territories. The areas of the anterior and middle cerebral arteries were designated as anterior, and the areas of the basilar artery, vertebral arteries, and posterior cerebral arteries were designated as posterior. The subtypes of ischemic stroke were classified using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) [18].
The IV thrombolytic therapy was carried out in compliance with international guidelines. Patients received tissue plasminogen activator (t-PA; Actilyse, Boehringer Ingelheim, Ingelheim, Germany) at a dose of 0.9 mg per kilogram of body weight (maximum, 90 mg), 10% of which was given as a bolus followed by delivery of the remaining 90% as a continual infusion over a period of 60 minutes [2]. In patients with systolic blood pressure > 180 mmHg or diastolic blood pressure > 110 mmHg, IV thrombolytic therapy was performed only after the high blood pressure was controlled by IV administration of labetalol.
For the evaluation of neurologic outcome, we reviewed the 24-hour NIHSS scores after administration of IV thrombolytic therapy as well as the NIHSS score upon patient discharge. The differences between these two scales and the initial NIHSS scores were described as ╬öNIHSS1 (difference of initial NIHSS and 24-hour NIHSS) and ╬öNIHSS2 (difference of initial NIHSS and NIHSS on discharge). When the NIHSS score increased after IV thrombolytic therapy, its value was documented as negative at a value of zero. In addition, early neurologic improvement (ENI) was defined as improvement of Ōēź 4 or 5 points in the NIHSS score and/or an NIHSS score of 0 at 24 hours and described as ENI-4/0 and ENI-5/0, respectively [3,19]. Major neurological improvement (MNI) was defined as an improvement of Ōēź 8 points in the NIHSS score and/or an NIHSS score of 0 at 24 hours and described as MNI-8/0 [3,20]. In addition, we investigated the presence of cerebral hemorrhage after IV thrombolytic therapy during the patientŌĆÖs admission period.
Statistical analysis was performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). First of all, a normality test was performed. If the variables showed a normal distribution, continuous variables were presented as mean and standard deviation. If variables were skewed, continuous variables were presented as medians with interquartile ranges and categorical variables presented as frequencies (percentages). Continuous variables were compared using the StudentŌĆÖs t-test or Mann-Whitney U-test according to the results of normality test and chi-square or FisherŌĆÖs exact test according to expected frequencies for categorical data. A P-value of < 0.05 was considered statistically significant.
RESULTSA total of 213 patients with acute ischemic stroke visited our ED during the study period. Of those, 163 patients were excluded for the following reasons: onset time > 3 hours (n=48), onset time < 3 hours but initial NIHSS score < 4 or NIHSS score improved to < 4 (n=73), disagreement with thrombolysis (n=1), inadequate medical record documentation (n=5), contraindication or no thrombolysis according to clinical judgment (n=6), had computed tomography scan only+IV thrombolysis ┬▒ additional intervention (n=19), and had MRI+IV thrombolysis+additional interventions (n=11). As a result, a total of 50 patients were included in this study (Fig. 1). Median age was 64.4 years (interquartile range, 54.5 to 78.0), and 29 patients (58.0%) were male. The DWI-FLAIR mismatch group included 36 patients (72.0%) and the DWI-FLAIR match group included 14 patients (28.0%).
Significant differences were not observed for age, gender, previous history of disease, current smoking status, current antiplatelet agent medications, vital signs, administration of antihypertensive drugs before IV thrombolytic therapy, initial NIHSS score, and time intervals (symptom onset to MRI examination, symptom onset to initiation of drug therapy, and MRI examination to initiation of drug therapy) between the two groups (Table 1). Moreover, there was no statistical difference in the location of cerebral ischemic stroke and the subtypes of TOAST between the two groups (Table 2).
There were, however, significant differences in ΔNIHSS1, ΔNIHSS2, ENI-4/0, ENI-5/0, and MNI-8/0 DWI-FLAIR between the two groups (Table 3). In particular, all 6 patients who showed increased NIHSS scores at 24 hours compared to the initial NIHSS score belonged to the DWI-FLAIR match group. Two patients also had a higher NIHSS score on discharge compared with the initial NIHSS scores; cerebral hemorrhage occurred in one of these patients.
DISCUSSIONDWI is a form of MRI that derives its image contrast from differences in the motion of water molecules between tissues. The diminishment of cerebral blood flow below a critical threshold leads to a disruption of energy metabolism, resulting in cytotoxic edema that can be depicted by a reduced apparent diffusion coefficient on DWI within minutes of a stroke. In contrast, FLAIR is an MRI sequence that produces strong T2-weighting combined with an inversion recovery pulse to suppress the signal that results from cerebral spinal fluid. DWI allows the detection of acute ischemic lesions within minutes of the ischemic cerebral event by showing high contrast, and FLAIR provides an advantage over the more conventional T2-weighted imaging for the detection of ischemic lesions, especially for lesions near the cerebral spinal fluid space.
Thirty-six patients (72.0%) showed a DWI-FLAIR mismatch in our study. In previous studies, a DWI-FLAIR mismatch identified patients within 3 hours of symptom onset with 71% to 97% sensitivity and 64% to 97% positive predictive value, and within 4.5 hours with 73% to 89% specificity and 83% to 97% positive predictive value [8,10-13]. According to a study by Aoki et al. [10], DWI-FLAIR mismatch was found in 14.8% of 162 ischemic stroke patients within 3 hours of symptom onset. Other studies have also suggested that acute ischemic stroke patients with DWI-FLAIR mismatch may be able to safely receive IV thrombolysis [21]. Of the 10 patients with anterior ischemic stroke and DWI-FLAIR mismatch who had neurologic changes documented within 3 hours after the ischemic event and received initial treatment within 3 to 24 hours after the last period of normal neurologic functioning, none showed any occurrences of cerebral hemorrhage, and 6 showed a neurological improvement of Ōēź 4 points on the NIHSS at 7 days after the ischemic event. It should be noted that Ebinger et al. [11] reported conflicting results regarding the effectiveness of thrombolysis in DWI-FLAIR mismatch versus match patients. Of 51 patients with a symptom onset time within 4.5 hours, 25 patients (49.1%) were in the DWI-FLAIR mismatch group, and neither lesion growth on DWI nor change on NIHSS at 24 hours differed significantly between the two groups.
As mentioned above, for the evaluation of neurologic outcomes, we reviewed the 24-hour and discharge NIHSS scores and calculated the difference between these two measurements and the initial NIHSS score. According to the National Institute of Neurological Disorders and Stroke rt-PA stroke study, there was no significant difference between the group given t-PA and the group given placebo among patients showing neurologic improvement (status improved to normal or by 4 or more points on NIHSS) at 24 hours, although a benefit was observed for the t-PA group at 3 months [3]. However, above a threshold of 5 points, the differences in the proportion of patients with ENI consistently were statistically in favor of rt-PA in the post-hoc analysis of the National Institute of Neurological Disorders and Stroke trial [19]. Brown et al. [20] reported that neurological improvement of Ōēź8 points in NIHSS scores or an NIHSS score of 0 at 24 hours was predictive of a favorable 3-month outcome. In our study, the patients with DWI-FLAIR mismatch showed a statistically significant improvement in the 24-hour and discharge NIHSS scores and on ENI-4/0, ENI-5/0, and MNI-8/0 as well. Although the neurologic outcomes at 3 months after onset were not reported in our study, MNI-8/0 was observed only in 10 of 36 patients with the DWI-FLAIR mismatch, and there was no increase in NIHSS scores or in the incidence of intracranial hemorrhage in the DWI-FLAIR mismatch group. Considering the fact that all enrolled patients had onset time within 3 hours and that there was no significant difference between the two groups in baseline characteristics, it is remarkable that 14 patients with DWI-FLAIR match had relatively poor neurologic improvement, and that 6 of those patients (42.9%), including one patient who experienced cerebral hemorrhage, showed neurological deterioration.
This study had several limitations. First, this study was retrospective. Second, the results of this study are difficult to generalize because of the small number of subjects. Third, the parameters of mid- and long-term prognosis, such as modified Rankin Scale or Barthel Index, were not reported. Fourth, this study did not include the patients who had onset time between 3 to 4.5 hours and who could have been candidates for IV thrombolytic therapy in accordance with the recently changed guideline. Although there may be other limitations of this study, we believe these results will help establish the clinical evidence that supports the use of IV thrombolytic therapy for acute ischemic stroke patients with unclear onset time and DWI-FLAIR mismatching.
REFERENCES1. Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr; American Heart Association Stroke Council. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009; 40:2945-8.
2. Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007; 38:1655-711.
3. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995; 333:1581-7.
4. George MG, Tong X, McGruder H, et al. Paul Coverdell National Acute Stroke Registry Surveillance-four states, 2005-2007. MMWR Surveill Summ 2009; 58:1-23.
5. Serena J, Davalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis 2003; 16:128-33.
6. Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002; 33:988-93.
7. Fisher M, Albers GW. Advanced imaging to extend the therapeutic time window of acute ischemic stroke. Ann Neurol 2013; 73:4-9.
8. Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4┬Ę5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 2011; 10:978-86.
9. Wu O, Schwamm LH, Sorensen AG. Imaging stroke patients with unclear onset times. Neuroimaging Clin N Am 2011; 21:327-44.
10. Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci 2010; 293:39-44.
11. Ebinger M, Ostwaldt AC, Galinovic I, et al. Clinical and radiological courses do not differ between fluid-attenuated inversion recovery-positive and negative patients with stroke after thrombolysis. Stroke 2010; 41:1823-5.
12. Petkova M, Rodrigo S, Lamy C, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology 2010; 257:782-92.
13. Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol 2009; 65:724-32.
14. Ebinger M, Galinovic I, Rozanski M, Brunecker P, Endres M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke 2010; 41:250-5.
15. Huisa BN, Liebeskind DS, Raman R, et al. Diffusion-weighted imaging-fluid attenuated inversion recovery mismatch in nocturnal stroke patients with unknown time of onset. J Stroke Cerebrovasc Dis 2013; 22:972-7.
17. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277:925-6.
18. Saposnik G, Fang J, Kapral MK, et al. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. Stroke 2012; 43:1315-22.
19. Haley EC Jr, Lewandowski C, Tilley BC. Myths regarding the NINDS rt-PA Stroke Trial: setting the record straight. Ann Emerg Med 1997; 30:676-82.
Fig.┬Ā1.Flow diagram. NIHSS, National Institutes of Health Stroke Scale; CT, computed tomography; MRI, magnetic resonance imaging. a)Intra-arterial thrombolysis or additional interventions were done in 7 of 48 cases. 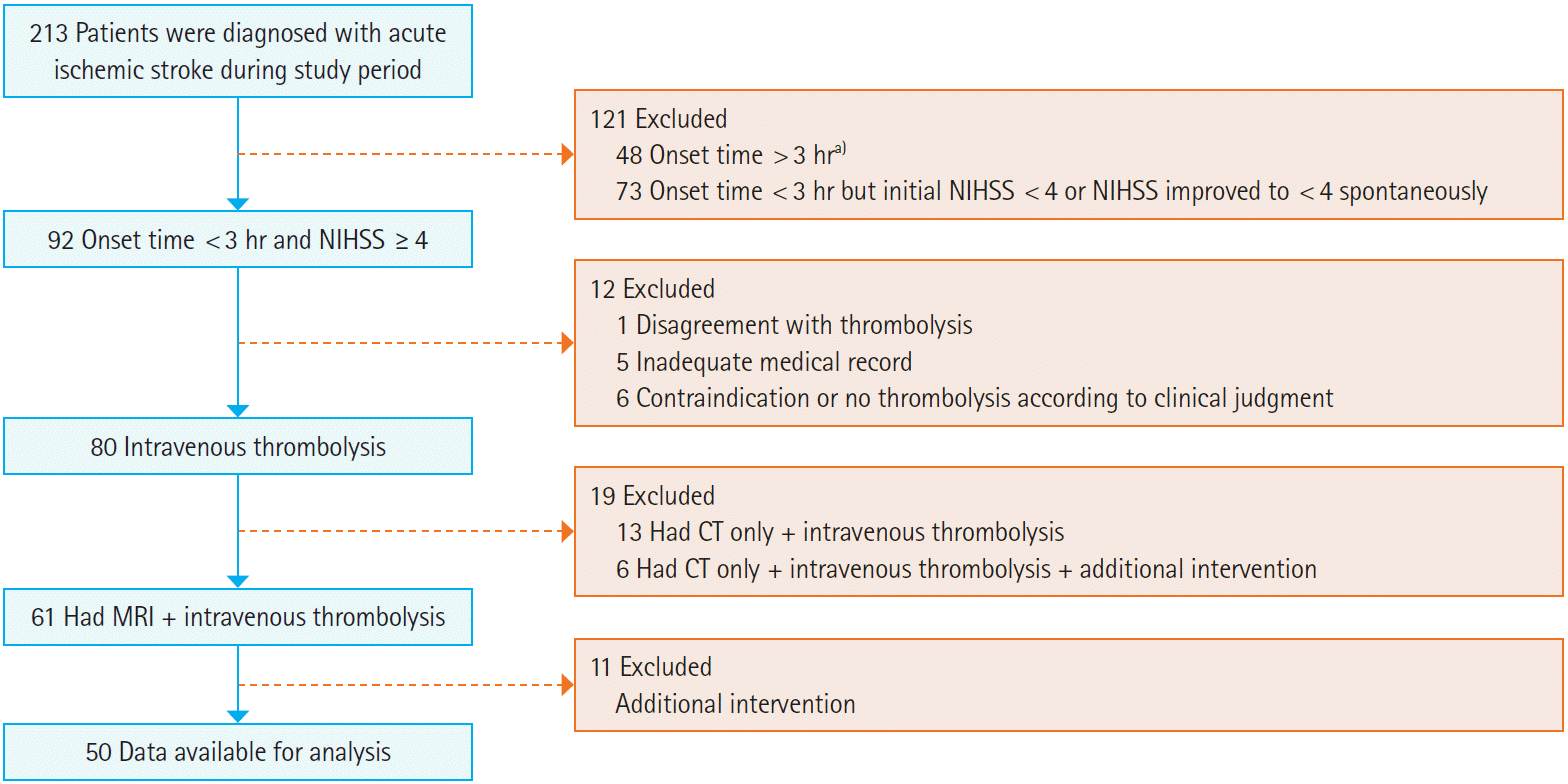
Table┬Ā1.Basic characteristics of the subjects
Values are presented as n (%), mean┬▒standard deviation, or median (interquartile range). DWI-FLAIR, diffusion-weighted imagingŌĆōfluid-attenuated inversion recovery; SBP, systolic blood pressure; DBP, diastolic blood pressure; NIHSS, National Institutes of Health Stroke Scale; DM, diabetes mellitus; MRI, magnetic resonance imaging; Drug, thrombolysis. Table┬Ā2.Classification of the ischemic stroke Table┬Ā3.Outcomes of thrombolytic therapy for acute ischemic stroke patients
Values are presented as median (interquartile range) or n (%). DWI-FLAIR, diffusion-weighted imagingŌĆōfluid-attenuated inversion recovery; NIHSS, National Institutes of Health Stroke Scale; ENI, early neurologic improvement; MNI, major neurologic improvement. a) Difference of National Institutes of Health Stroke Scale (NIHSS) scores obtained initially and 24 hours later. d) NIHSS score 24 hours after thrombolysis dropped to 0 or dropped 4 points from the initial NIHSS score. |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||