AbstractPoint-of-care ultrasound (POCUS) is a rapidly developing technology that has the potential to revolutionize emergency and critical care medicine. The use of POCUS can improve patient care by providing real-time clinical information. However, appropriate usage and proper training are crucial to ensure patient safety and reliability. This article discusses the various applications of POCUS in emergency and critical care medicine, the importance of training and education, and the future of POCUS in medicine.
INTRODUCTIONPoint-of-care ultrasound (POCUS) is an ultrasound examination performed and interpreted by the clinician at the bedside to obtain specific clinical information. Although first used by clinicians in the 1960s, POCUS had not become increasingly popular in the 1990s, which subsequently resulted in it being labeled the “visual stethoscope” of the 21st century [1,2].
POCUS exam had been designed to address a specific clinical inquiry with a focused, goal-directed evaluation. Its objective is to either “rule in” or “rule out” specific conditions or answer a “yes or no” question [3–5]. By contrast, a comprehensive ultrasound (standard consultative ultrasound) performed by radiologists or cardiologists thoroughly evaluates the entire anatomical region. Ordering, executing, interpreting, and reporting such comprehensive ultrasound examinations typically take hours or days, whereas POCUS examinations provide clinical information in real-time within minutes. Recent studies have shown that POCUS can increase diagnostic accuracy and significantly reduce physicians’ diagnostic uncertainty [6]. Moreover, most patients admitted to an emergency department who agreed to undergo POCUS of the heart, lungs, and deep veins reported “very low” discomfort [7].
Clinicians have also released statements advocating for POCUS [8,9]. Additionally, undergraduate medical students who have encountered POCUS examinations earlier in their medical education have gained a better understanding of the clinical applications of POCUS [10]. POCUS has become increasingly popular in emergency medicine (EM), and so has POCUS education in residency programs [11].
With the increased use of ultrasound in emergency and critical care settings, countries with emergency rooms and intensive care units (ICU) equipped with an ultrasound system have recently implemented health insurance coverage for POCUS in emergency and critical care areas. Ultrasound practice is facilitated by standardized scopes and indications of use recommended by each country [12]. Although POCUS has been a rapidly growing technology in emergency, trauma, and critical care medicine, some concerns have been raised regarding its patient safety, which include overuse, inaccurate diagnoses, inappropriate usage, and excessive dependence on POCUS [13,14]. To improve patient care and prevent unnecessary cuts in healthcare budgets, proper prescription, application of POCUS, as well as documentation of its findings, are required [15].
This paper aims to comprehensively review the different types of POCUS used in clinical practice for emergency and critical care medicine, so that clinicians performing POCUS can better understand POCUS indications and limitations.
POCUS ULTRASOUND: EQUIPMENT AND INSTRUMENTATIONThe miniaturization of ultrasound machines and the increase in computing capacity have facilitated the development of portable ultrasound devices [16–18]. Currently, a vast selection of ultrasound devices is available in the POCUS market [19]. Given the developments in signal computational capacity, even the smallest mobile devices now provide high-quality images. Moreover, the price of POCUS has decreased dramatically, making the technique more accessible to physicians.
Several types of ultrasound machines have been used for POCUS, which can be categorized as “compact cart-based,” “handcarried,” and “handheld or pocket-sized” (Fig. 1). Compact cartbased devices are designed to be brought to the patient’s bedside. They possess the most advanced features with powerful processors and have the largest screen size and memory. However, these devices have some disadvantages including their large size, less maneuverability, indoor use only, short battery time, and high cost. Hand-carried ultrasound machines have a clamshell laptop or tiny television design, with recent devices using touchscreen displays. They are relatively lightweight, which allows for easy hand-carrying. Additionally, they have an extended battery time life and are capable of producing high-quality images. However, they have fewer advanced features and lesser storage space or transducer attachments than cart-based types. Pocket-sized machines are the most portable devices with the lightest weight, longest battery time, and lowest cost.
However, they produce relatively low-quality images and possess fewer features or workflow processes. Although this market is still very nascent, it is developing quite rapidly. New machines provide a mobile application-based system in which a tablet or smartphone may be transformed into a portable ultrasound by connecting a probe directly or wirelessly (Fig. 2).
The practice environment needs to be considered when choosing an ultrasound device. Cart-based devices may perform best in an emergency department or ICU but not outside of the hospital. In contrast, pocket-sized devices, while convenient and easy to use, may have limited features for in-hospital situations. Future technology development would provide a more cost-effective POCUS device.
FOCUSED CARDIAC ULTRASOUNDFocused cardiac ultrasound (FoCUS) is used to rapidly assess cardiac anatomy and function in critically ill patients at the bedside. The five basic views recommended for FoCUS examination include the parasternal long axis, parasternal short axis, apical four chamber, subcostal four chamber, and subcostal inferior vena cava (IVC) views [20,21]. Grayscale ultrasound is used to evaluate cardiac structure, during which depth and gain adjustments should be set for optimal visualization. Color Doppler analysis of the mitral and aortic valves to identify regurgitation may be included in the examination [6,22]. Assessments with FoCUS can identify several causes in time-sensitive clinical scenarios related to cardiorespiratory symptoms and signs (Table 1) [15,21,23,24].
Limitations and special considerations of FoCUSThe limitations of FoCUS related to patients include body habitus, surgical dressing or chest drains, and subcutaneous emphysema, which may increase the difficulty of obtaining clear images during a FoCUS examination. False-positive or false-negative results may occur due to off-axis viewing when scanning is not performed in the appropriate position for optimal image acquisition. Additionally, FoCUS has limitations in verifying some cardiac conditions such as pericardial fat pads, cysts, preexisting or small pericardial fluid, diastolic dysfunction, valvular diseases, and pulmonary hypertension [25]. Comprehensive echocardiography or additional diagonal modalities should be considered for a complete evaluation of uncertain findings or complex clinical presentations.
To perform FoCUS accurately and effectively, proper education and training are required. FoCUS training typically includes a combination of didactic instruction, hands-on training, and supervised clinical experience [26]. Trained physicians should be able to demonstrate proficiency in obtaining and interpreting FoCUS images before performing the examination independently. Continuous quality improvement and education are important to maintain and improve the accuracy and reliability of FoCUS.
THORACIC ULTRASOUNDThoracic ultrasound has been increasingly used in the management of patients who visit the emergency department due to acute dyspnea and respiratory failure. This is due to its effectiveness in aiding decision-making for differential diagnosis and treatment. Unlike simple radiography and computed tomography (CT), thoracic ultrasound can be quickly and safely applied to patients, making it an effective choice for early imaging examinations [27,28].
Clinical application of thoracic ultrasoundSince Lichtenstein and Meziere [29] announced the BLUE (Bedside Lung Ultrasound in Emergency) protocol in 2008, experts [21,30] have suggested the use of lung ultrasound for pneumothorax, alveolar-interstitial syndrome, pulmonary consolidation, pleural effusion, and neonates and pediatrics, with robust evidence and strong recommendations in 2012.
Normal lung patternWhen a normal lung pattern, characterized by a bat sign, A line, and lung sliding are observed on lung ultrasound, pneumothorax and interstitial syndrome can be excluded (Fig. 3A). However, when a normal lung pattern is observed in patients who complain of acute dyspnea or breathing difficulties, airway diseases such as asthma or chronic obstructive pulmonary disease, may be considered to have caused their condition and not problems with the lung tissue itself.
PneumothoraxThe absence of lung sliding may indicate pneumothorax, which may be confirmed through lung point observation (sensitivity, 91%; specificity, 98%) (Fig. 4) [30]. However, care should be exercised for tension pneumothorax, including the whole pleural cavity, given that a lung point cannot be observed in such cases. Checking the lung pulse and B-lines can ultimately exclude pneumothorax.
Interstitial syndromeInterstitial syndrome can be diagnosed through the presence of more than three B lines in the intercostal space (sensitivity, 94%; specificity, 92%) [31]. Although pulmonary edema due to heart failure is the most common clinical cause of interstitial syndrome, various other heart and lung diseases have been shown to cause the same condition (Fig. 3B).
ConsolidationThe most common clinical cause of consolidation is pneumonia. Given the various locations and forms of pneumonia, various presentations can also be observed on thoracic ultrasound (Fig. 3C). Subpleural consolidation and tissue-like patterns are typical and can be diagnosed even if a shred sign or effusion is visible on the posterior examination (sensitivity, 94%; specificity, 96%) [32].
EffusionPleural effusion can be easily diagnosed on thoracic ultrasound, which is superior to radiography and CT in measuring the quantity and predicting the properties of effusion. Real-time ultrasound guidance can also help physicians safely perform procedures, such as thoracentesis.
The BLUE protocolThe BLUE protocol is a fast and accurate bedside lung ultrasound technique for diagnosing acute respiratory failure. It involves scanning three points on each hemithorax and identifying specific ultrasound signatures. The BLUE protocol is part of a larger framework for critical care ultrasound [33].
ABDOMINAL POCUSAbdominal POCUS can be performed in any patient with abdominal symptoms complaining of abdominal pain, flank pain, and a distended abdomen. The advantages of POCUS include rapid performance, avoidance of unnecessary radiation and contrast exposure, quick diagnosis, and potential reduction in length of hospitalization and costs [34–38].
Clinical applications of abdominal ultrasoundRight upper quadrant of the abdomenPOCUS for right upper quadrant (RUQ) pain is a useful tool for evaluating acute cholecystitis. The most significant positive findings are the sonographic Murphy sign, the presence of cholelithiasis, gallbladder wall thickening, and pericholecystic fluid collection. Dilatation of the common bile duct can also be identified in the RUQ areas. Recent studies have suggested that POCUS performed by EM physicians and radiologists had similar accuracy in detecting acute cholecystitis [39,40].
Renal and aortic ultrasoundRenal POCUS aids EM physicians in detecting hydronephrosis in renal colic patients. Additionally, bladder ultrasound helps detect ureterovesical junction or bladder stones, and the absence of ureteral jet in patients suspected to have obstructive uropathy [41,42]. POCUS of the aorta includes measuring the diameter of the abdominal aorta and inspecting for the presence of an intimal flap in the case of aortic dissection using both the transverse and longitudinal ultrasound planes. Moreover, aortic ultrasound can help emergency physicians identify a ruptured abdominal aortic aneurysm (AAA) with high sensitivity and specificity [43,44].
Various gastrointestinal diseasesPOCUS is a valuable tool for diagnosing various gastrointestinal pathology. POCUS findings for small bowel obstruction include increased loop dimensions, increased or decreased peristaltic movements (to-and-fro sign), and enlarged and visible valvulae conniventes (keyboard sign) [45,46]. In patients with right lower quadrant abdominal pain, a noncompressible tubular structure with a target sign greater than 6 mm in diameter at the site of the appendix is suggestive of acute appendicitis [47]. In women of reproductive age, pelvic pain or lower abdominal pain can be caused by ovarian torsion or ovarian cyst rupture. Due to the difficulty in diagnosing ovarian torsion or ovarian cyst rupture based on symptoms and physical examination alone, POCUS has become the primary modality for its evaluation [48].
ULTRASOUND-GUIDED PROCEDURESUltrasound guidelines, including those from the American College of Emergency Physicians (ACEP) and other organizations, should incorporate guidance on procedures considering the integral role these procedures play in patient management, as well as in enhancing the safety and efficacy of interventions [49]. This improves ultrasound technology has widened the scope of guidelines beyond disease diagnosis and management to include procedural guidance to ensure optimal patient care in every setting.
In critically ill patients, bedside needle procedures such as central venous catheter insertion, thoracentesis, and pericardiocentesis are frequently required. Ultrasound-guided procedures have significant advantages over landmark-based approaches [50–54]. The increasing availability of ultrasound machines, and portable devices, as well as the continued emphasis on patient safety in critical care, have contributed to the growing utilization of ultrasound-guided procedures.
Ultrasound-guided techniques can be classified into two categories: static and dynamic. The static technique involves using ultrasound to locate a target structure and fix the medical instrument, whereas the dynamic technique enables continuous visualization of the needle or instrument as it progresses toward the target. Both approaches have advantages and disadvantages.
Ultrasound probes can be positioned in three axes: short (perpendicular to the structure’s long axis), long (parallel to the structure’s long axis), and oblique (at an angle to the long axis) [55–57]. Each axis provides a different view for visualization, with the short, long, and oblique axis offering cross-sectional, lengthwise, and angled perspectives, respectively.
After positioning the probe in an axis, two techniques are commonly used: in-plane and out-of-plane. The in-plane technique aligns the needle or instrument with the ultrasound probe, allowing direct visualization of both the instrument and the target structure on the same ultrasound image. Although considered accurate and safe, this technique requires precise needle positioning. The out-of-plane technique positions the needle or instrument in a different plane from the probe, resulting in separate visualization of the needle and target structure [58,59].
The choice of technique could depend on specific situations and practitioner preference. However, practitioners need to be proficient in all techniques to ensure optimal outcomes [60]. Micropuncture needles (21 gauge) have become popular in EM and critical care due to their smaller size, reduced bleeding risk, and improved tolerance for multiple vessel wall punctures [61].
TRANSCRANIAL ULTRASOUNDPatients with altered consciousness should undergo CT or magnetic resonance imaging to rule out intracranial lesions. However, these imaging modalities are not always available, and transporting patients with unstable vital signs for radiologic studies may be challenging. In those situations, immediate and noninvasive bedside tests are imperative.
Transcranial ultrasound (TUS), which was first introduced in 1982 had subsequently become a routine clinical procedure for qualitatively and noninvasive evaluation of intracranial blood flow [62]. The development of B-mode ultrasound has helped clinicians evaluate the brain parenchyma. Despite providing inferior quality images compared to CT, ultrasound is capable of providing sufficient image quality for appropriate clinical management, such as the detection of acute hematomas and midline shifting due to the mass effect in the brain parenchyma [63].
The TUS examination is performed via the temporal approach with a low frequency phased array transducer. The main structures needed to be identified on the screen include the contralateral temporal bone at the bottom of the screen and the butterfly-shaped midbrain in the center of the screen. Lastly, the third ventricle should be identified when the probe is slightly tilted.
In general, two types of brain lesions should be easily noticeable through TUS [64]. The first is acute hematoma, which appears as an echo-enhancing mass within the brain parenchyma (Fig. 5). The second is a midline displacement in which the third ventricle is displaced in the opposite direction due to the mass effect (Fig. 6). However, given that the skull becomes thicker with age, resulting in the attenuation of ultrasound waves, insufficient image qualities are obtained in approximately 5% to 20 % out of patients [65]. Although recent attempts have been made to increase the diagnosis rate using contrast agents, future studies will be necessary to overcome these limitations [66].
POCUS IN CARDIORESPIRATORY ARRESTDuring cardiac arrest, POCUS is primarily utilized to guide procedures, immediately identify and treat reversible causes, monitor the quality of chest compression, and predict outcomes [67,68]. POCUS can be considered as an additional evaluation method in cases when the operator is highly skilled and its use does not impede chest compressions [68,69].
Although quantitative capnography has been considered the gold standard for confirming endotracheal tube placement, its sensitivity decreases in prolonged cardiac arrest [70–72]. Thus, physicians have attempted to use POCUS to confirm endotracheal tube placement, with their findings showing reliable accuracy [73–76]. In particular, real-time confirmation showed higher accuracy.
Central venous access can be considered in cases where intravenous access through subcutaneous veins is difficult or extracorporeal cardiopulmonary resuscitation is required [77]. Conventional central venous access has several disadvantages, including interruption of cardiopulmonary resuscitation, technical difficulty, and several complications [78]. Ultrasound-guided central venous access can greatly increase stability, accuracy, and efficiency [79–81]. Ultrasound-guided supraclavicular subclavian access using an endocavitary probe improves vein identification, anatomical understanding, and procedural comfort following a brief training session [82].
POCUS can help determine cardiac tamponade, left ventricle failure, pulmonary embolism, hypovolemia, and tension pneumothorax [83,84]. Several protocols have been established to identify the causes of cardiac arrest (FATE [Focused Assessed Transthoracic Echocardiography], FEER [Focused Echocardiographic Evaluation in Resuscitation], CAUSE [Cardiac Arrest Ultra-Sound Exam], SESAMI [Sonography in Shock and Acute Management in Intensive Care], ShoC [Sonography in Hypotension and Cardiac Arrest], etc.) [85–89]. POCUS is best performed in the subxiphoid or apical window to avoid interfering with chest compressions. The BLUE and PLAPS (Posterior and/or Lateral Alveolar and/or Pleural Syndrome) points are used to evaluate the lung, whereas a subcostal window is used to evaluate the inferior vena cava. Finally, proximal leg veins are scanned to confirm deep vein thrombosis, and Focused Assessment with Sonography in Trauma (FAST) is performed to identify sources of blood loss.
Some physicians have attempted to determine the prognosis of cardiac arrest using POCUS. Especially, they found that pulseless electrical activity (PEA) without cardiac activity was associated with a worse prognosis than PEA with cardiac activity. Moreover, the likelihood of return of spontaneous circulation (ROSC) was considerably very low in the absence of cardiac activity on serial POCUS [84,90]. However, nonserial POCUS demonstrated poor performance in predicting ROSC.
To monitor chest compression quality, transesophageal echocardiography (TEE) can be a good option. However, TEE requires performers to be highly trained [67,91]. Recently, attempts have been made to monitor chest compression quality through TTE (subcostal or apical window) [92].
More studies are needed to obtain evidence in support of including POCUS as a part of the resuscitation algorithms [93].
POCUS IN THE DIAGNOSIS AND MANAGEMENT OF SHOCKPOCUS can provide real-time physiologic information for patients in shock. This approach can also be useful in identifying the cause of shock, classifying the type of shock, guiding treatment, and evaluating the response to therapy. Multifocal ultrasonography can be used for the initial evaluation of undifferentiated shock to identify the cause and provide prompt treatment [94].
When using ultrasonography in shock, the main goals should be to assess the presumed etiology and category of shock and monitor the real-time response to management to optimize treatment efforts. Practical tips for using ultrasonography include performing basic examinations of the heart, lungs, and IVC to recognize the category of shock, as well as supplementary examinations of free fluid in the abdomen, abdominal aorta, intra-abdominal solid organs, peripheral vessels, and other areas to evaluate the cause of shock (Table 2).
Examinations should be performed using multiorgan views and windows. Accordingly, cardiac examination should assess left and right ventricular size and function, pericardial effusion, gross regional wall motion abnormalities, and gross valvular abnormalities. Thoracic examination should evaluate pleural effusion, B-lines, and lung sliding. Vasculature examination should assess the size and collapsibility of the IVC, aorta, and femoral/popliteal veins.
The key ultrasound findings associated with shock are summarized in Fig. 7. Several protocols have been developed for the evaluation of patients in shock, including the RUSH (Rapid Ultrasound in Shock) protocol [95,96], SHoc (Sonography in Hypotension and Cardiac Arrest) protocol [89], ACES (Abdominal and Cardiac Evaluation with Sonography in Shock) [97], FATE [85], and FEEL (Focused Echocardiography Evaluation in Life Support), among others. These protocols include assessments of cardiac function, volume, and vasculature [83].
EXTENDED FASTFAST examination is a noninvasive point-of-care test that aims to guide clinical decision-making and direct angiographic or surgical interventions. It can be a powerful tool for clinicians, especially in time-dependent situations such as trauma. Extended FAST (eFAST) is an evolution of the traditional FAST that incorporates thoracic window assessment to identify hemothorax and pneumothorax [98–100]. The physiologic priority of ABCD (airway, breathing, circulation, and disability) in injured patients should be assessed using a multisystem, multifocused, problem-based POCUS as an extension of physical examination [101,102]. This ultrasound-enhanced trauma life support, called FAST-ABCD, can provide a considerable amount of important information that could help the primary physician make critical decisions by systemically combining airway, lung, cardiovascular, abdominopelvic, orbital, and transcranial ultrasound findings [103–106].
Additionally, this approach can help determine if the airways are open and guide procedures like endotracheal intubation and cricothyroidotomy. It can also provide information on lung contusion and limited hemodynamics. Furthermore, it aids in the differential diagnosis of shock and intracranial hypertension. This method even allows for an extensive secondary survey from head to toe [103,105]. The indications for the utility of ultrasound in trauma continue to evolve beyond FAST [106]. FAST-ABCD can be incorporated into advanced trauma life support [107,108]. Evaluation using eFAST can provide critical information during the real-time assessment of patients with complex trauma.
POCUS IN PEDIATRIC CAREUltrasonography offers real-time imaging without ionizing radiation, making it a safe and effective option in pediatric care [109]. The smaller body sizes, less fat, and thinner abdominal walls of children allow for high-resolution images to be obtained using ultrasonography, which can be used for various diagnostic and procedural applications in pediatric emergency care.
Clinical applications of POCUS in pediatric careLung and cardiac ultrasoundUltrasonography is a highly effective diagnostic tool for diagnosing pneumonia in pediatric patients [110], with available evidence showing its high sensitivity and specificity in identifying pneumonia by detecting B-lines, parenchymal consolidation, and pleural effusion. Similar to that in adults, ultrasonography can be used to diagnose pneumothorax in pediatric populations. Focused echocardiography can detect pericardial effusion and tamponade, assess global contractility, and evaluate left ventricular function and right ventricular filling [111]. Patients with severely impaired cardiac function on focused echocardiography may be suspected to have myocarditis.
Abdominal and testicular ultrasoundUltrasonography can identify the underlying causes of vomiting or acute abdominal pain, which are common symptoms in emergency pediatric patients. Serious diseases such as pyloric stenosis, midgut volvulus, intussusception, and appendicitis can be immediately diagnosed using ultrasound. Furthermore, even novice sonographers can achieve high diagnostic rates for intussusception [112]. In cases of testicular swelling or pain, ultrasound can differentiate between various conditions, such as testicular torsion, torsion of the appendix testis, epididymo-orchitis, and hydrocele. Ultrasonography can be used to determine the structure and vascularity of the testis. A decrease in the blood flow to the testicles indicates testicular torsion [113].
POCUS in traumaThe eFAST has been routinely performed for a long time in patients with chest and abdominal trauma. Unlike in adults, its diagnostic usefulness is limited in children, such that a negative eFAST result in children may not accurately rule out the presence of intra-abdominal injuries [114].
Extremities ultrasoundUltrasound can be used to detect hip joint effusion in children who visit the hospital with a limping gait. Septic arthritis and transient synovitis can be differentiated through fluid analysis by performing fluid aspiration using ultrasound. Ultrasound can also be a helpful diagnostic tool in detecting fractures in children. Although radiography has been the primary imaging modality used for assessing trauma, ultrasound can be useful for evaluating unossified structures, fractures extending to the unossified epiphyses, occult fractures, physeal separation, intra-articular bodies, ligamentous injuries, and occasionally periosteum trapped between fracture fragments [115].
Clinical applications in ultrasound-guided proceduresUltrasound-guided procedures enhance the success rate and safety of central venous catheter placements [116,117]. It’s also beneficial for securing peripheral blood vessels, detecting foreign bodies, and performing joint aspirations. When a child gets a splinter or other small foreign body embedded in their skin, ultrasound can help locate the foreign body and guide its removal, reducing the need for more invasive procedures [118].
POCUS IN EMERGENCY CARECharacteristics of emergency department patientsEmergency department (ED) patients present with a wide range of problems, from critical emergencies to minor issues that can be easily treated. However, we currently lack an advanced medical system to accurately diagnose all patients immediately upon arrival.
Several patients in the ED express their concerns in a disorganized manner. Some patients are unable to communicate due to changes in their level of consciousness. Emergency physicians (EP) are trained to gather information from a brief history and physical examination, interpret it as clinical manifestations, and form a hypothesis.
POCUS IN EDPOCUS has become a crucial component of emergency medicine given its ability to improve physical assessments and provide critical information to support or exclude hypotheses at the bedside while awaiting laboratory and imaging results. Attending EPs who appreciate the clinical features of the patient and perform POCUS can effectively enhance patient flow and reduce errors.
The role of EP and POCUSThe role of the EP can be distilled into three primary tasks: stabilization, differential diagnosis, and monitoring (of patient status and procedure). EPs are required to differentiate between critical and noncritical emergencies, stabilize patients, conduct a comprehensive evaluation for differential diagnosis, and determine the most suitable initial treatment. Furthermore, they need to monitor the effectiveness of treatments and utilize ultrasound guidance for procedures. POCUS aids EPs in all these tasks by enhancing safety and accuracy, minimizing errors, and facilitating faster patient flow in the ED [119].
The scope of practice for EM POCUSIn 2009, the ACEP established guidelines for EM POCUS, outlining its scope of practice, which included 11 core applications and five functional clinical categories [120]. These categories encompass resuscitative measures, diagnostic and symptom-based assessments, procedure guidance, and therapeutic monitoring (Fig. 9). Substantial evidence supports the use of multiorgan POCUS for evaluating and managing patients with cardiopulmonary or hemodynamic failure. POCUS using established protocols, can provide crucial information for swift diagnosis and appropriate management in critical situations that demand immediate attention and intervention to prevent death or serious harm. Such critical situations include cardiac arrest [86,87,121], major torso trauma [99,122], shock [95,97,123,124], respiratory difficulty [29,125,126], chest pain [127–129], and life-threatening abdominal pain. Noncritical situations such as scrotal pain, ocular symptoms, soft tissue or musculoskeletal problems, and noncritical abdominal pain also fall within the scope of EM POCUS (Figs. 10, 11).
While it may be challenging for EPs to provide optimal care for each patient without causing harm, their proficiency with POCUS would undoubtedly enhance patient flow and reduce errors.
POCUS IN CRITICAL CAREPOCUS is commonly used in critical care settings, primarily in the ICU where assessments should be comprehensive rather than urgent. Given that POCUS examinations do not cover the full functional and structural status of patients, comprehensive ultrasound has been commonly used in the ICU. Additionally, it may be challenging for ICU clinicians to master all the specialized and professional skills across various fields. Comprehensive echocardiography requires considerably more image acquisition, proficiency, and experience than POCUS [130]. Thus, ICU clinicians determine whether ultrasound evaluation is needed and link clinical departments with experts.
Many ICU clinicians are well-trained to perform specialized ultrasound studies. Several coronary care intensivists perform comprehensive echocardiography, whereas a few respiratory care intensivists learn lung ultrasound. Hence, ICU clinicians can diagnose critically ill patients quickly and accurately with their acquired abilities. A POCUS study in an ICU is dependent on the skill and experience of the clinician performing the study. Therefore, the scope of examination can vary based on both the clinician and patient admitted to the ICU [24]. Despite comprehensive ultrasound study, pleural effusion, pneumothorax, ascites, and deep vein thrombosis is frequently diagnosed using POCUS [131].
Additionally, intensive care patients may experience sudden exacerbations of preexisting diseases or complications. ICU clinicians should be ready to evaluate and manage any hemodynamic and respiratory deteriorations using POCUS. Regardless of the type of ICU or disease being treated, POCUS is crucial for assessing cardiac function, cardiac tamponade, significant valvular dysfunction, and determining the fluid responsiveness of patients in shock [24]. Given that the heart pumps and beats constantly, assessing its function via the ultrasound can be quite challenging. Acquiring proficiency in echocardiography demands a significant investment of time and effort [131].
Catheterization should always be conducted in an aseptic and safe manner under ultrasound guidance [132]. Furthermore, due to the severity of their conditions, ICU patients often have limited transportation. As a result, drainage catheters are frequently inserted into various thoracic and abdominal organs at the bedside.
POCUS EDUCATION CURRICULUM (STUDENTS AND RESIDENTS)Although academic hospitals have increasingly adopted POCUS, there remains a wide variability in residency training. The ACEP published the first guideline for emergency ultrasound in 2001 [120], which had been subsequently updated in 2009 and 2014. On the other hand, the International Federation for Emergency Medicine (IFEM) released its POCUS guidelines in 2015 [129]. Both guidelines share similar applications and educational content, but their scope and methods differ. Educational guidelines suitable for medical practice are needed in Korea.
ACEP policy statementThe ACEP categorizes emergency ultrasound into five clinical areas: resuscitation, diagnosis, symptoms, procedure guidance, and therapeutic monitoring. There are 12 core applications for POCUS, including trauma, intrauterine pregnancy, AAA, cardiac, biliary, urinary tract, deep vein thrombosis, musculoskeletal and nerve, thoracic, ocular, bowel, and procedural guidance. The ACEP advocates for an educational curriculum that underscores the reasons for performing emergency ultrasounds and offers hands-on experience [49]. It is crucial to establish standardized training and education curricula for POCUS in areas such as scope of practice, competency training, hospital credentialing, specialty certification, quality control, and leadership in clinical ultrasound [120].
IFEM POCUS curriculumThe IFEM has established guidelines for POCUS training programs, categorizing applications into core and enhanced clinical applications. They emphasize initial introductions through short lectures and gaining experience in imaging acquisition, interpretation, and clinical integration [133].
POCUS training in undergraduate medical educationThe first national survey of undergraduate medical education in the United States revealed that ultrasound instruction was offered in 62% of medical schools, predominantly in the third year [134]. According to the most recent national survey in 2020, 69 medical schools have integrated the POCUS into their training curriculum [135]. A study that implemented a 4-year ultrasound curriculum found that its graduates relied more heavily on POCUS than their peers in their respective specialties, and that their POCUS findings often influenced their case management [136].
POCUS training of EM in KoreaIn 2021 and 2022, the Society of Emergency and Critical Care Imaging (SECCI) conducted a Delphi survey of 50 specialists in emergency and critical care medicine who used POCUS to identify POCUS applications they should incorporate into their education. The survey showed that domestic guidelines should follow international trends. However, further research is required to ascertain the appropriate level of skill trainees should achieve at different stages of their training.
Beyond acknowledging the significance of POCUS in emergency medical settings, basic ultrasound education also needs to be considered. Several educational methods and guidelines are being developed. At this point, we believe it necessary to establish guidelines for standardizing education in Korea.
FUTURE PERSPECTIVES AND BEYONDCurrently, POCUS helps physicians diagnose and treat patients in real-time. Advances in POCUS have revolutionized EM, critical care, and severe trauma.
Beyond EDs, ICUs, and trauma centersIn various medical settings, POCUS could revolutionize diagnosis and treatment. In the future, POCUS may be applied to primary care clinics, ambulatory surgery centers, patients’ homes as well as EDs, ICUs, and trauma centers. As handheld devices become more advanced and lighter, they will become increasingly useful in areas such as prehospital emergency medicine, disaster relief, and war zones, where access to healthcare facilities may be limited.
Future perspectivesPOCUS has become a valuable tool for healthcare providers to perform rapid assessments in clinical settings, particularly for neonates and children who may not have easy access to conventional imaging. In many emergency and critical care situations, this device provides real-time imaging and diagnostic capabilities near the patient’s bedside, aiding in prompt and accurate diagnosis [137]. As handheld devices become more advanced and lighter, and as equipment and enhanced training programs become available, POCUS is expected to become an integral part of early patient care.
CONCLUSIONPOCUS is a versatile and valuable tool in emergency and critical care medicine. It provides real-time clinical information and enhances diagnostic accuracy. With the development of portable ultrasound devices, accessibility has expanded. Various applications of POCUS have demonstrated significant clinical benefits. However, appropriate usage and proper training are crucial to ensure patient safety and reliability. Guidelines and educational curricula are essential for standardizing POCUS training. As technology and training programs continue to advance, the effectiveness of POCUS will only increase.
NOTESCONFLICT OF INTEREST
Yong Soon Cho and Je Hyeok Oh are Editorial Board members of Clinical and Experimental Emergency Medicine, but were not involved in in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
REFERENCES1. Sekiguchi H. Tools of the trade: point-of-care ultrasonography as a stethoscope. Semin Respir Crit Care Med 2016; 37:68-87.
2. Goldberg BB, Gramiak R, Freimanis AK. Early history of diagnostic ultrasound: the role of American radiologists. AJR Am J Roentgenol 1993; 160:189-94.
3. Cardim N, Fernandez Golfin C, Ferreira D, et al. Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination. J Am Soc Echocardiogr 2011; 24:117-24.
4. Gorcsan J 3rd, Pandey P, Sade LE. Influence of hand-carried ultrasound on bedside patient treatment decisions for consultative cardiology. J Am Soc Echocardiogr 2004; 17:50-5.
5. Beaton A, Aliku T, Okello E, et al. The utility of handheld echocardiography for early diagnosis of rheumatic heart disease. J Am Soc Echocardiogr 2014; 27:42-9.
6. Shokoohi H, Boniface KS, Pourmand A, et al. Bedside ultrasound reduces diagnostic uncertainty and guides resuscitation in patients with undifferentiated hypotension. Crit Care Med 2015; 43:2562-9.
7. Laursen CB, Sloth E, Lassen AT, et al. Does point-of-care ultrasonography cause discomfort in patients admitted with respiratory symptoms? Scand J Trauma Resusc Emerg Med 2015; 23:46.
8. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med 2019; 14:E1-6.
9. Lewis D, Rang L, Kim D, et al. Recommendations for the use of point-of-care ultrasound (POCUS) by emergency physicians in Canada. CJEM 2019; 21:721-6.
10. Coiffier B, Shen PC, Lee EY, et al. Introducing point-of-care ultrasound through structured multifaceted ultrasound module in the undergraduate medical curriculum at the University of Hong Kong. Ultrasound 2020; 28:38-46.
11. Gottlieb M, Arno K, Kuhns M, Chan TM. Distribution of clinical rotations among emergency medicine residency programs in the United States. AEM Educ Train 2018; 2:288-92.
12. Chung SH, Lee HJ, Kim HS, Oh JY. Health insurance benefit criteria and quality assurance policies of diagnostic ultrasound services in other countries. Health Policy Manag 2014; 24:109-19.
13. Su E, Soni NJ, Blaivas M, Bhargava V, Steffen K, Haileselassie B. Regulating critical care ultrasound, it is all in the interpretation. Pediatr Crit Care Med 2021; 22:e253-8.
14. Blanco P, Volpicelli G. Common pitfalls in point-of-care ultrasound: a practical guide for emergency and critical care physicians. Crit Ultrasound J 2016; 8:15.
15. Choi WJ, Ha YR, Oh JH, et al. Clinical guidance for point-ofcare ultrasound in the emergency and critical care areas after implementing insurance coverage in Korea. J Korean Med Sci 2020; 35:e54.
16. Frentzel-Beyme B. From echo-sounding to color doppler sonography: the history of diagnostic ultrasonic diagnosis. Radiologe 2005; 45:363-70.
17. Eckel H. Sonography in emergency diagnosis of the abdomen (author’s transl). Rontgenblatter 1980; 33:244-8.
18. Plummer D. Principles of emergency ultrasound and echocardiography. Ann Emerg Med 1989; 18:1291-7.
19. iData Research Inc. Versatile point of care ultrasound technology quickly gaining market share in the United States [Internet]. GlobeNewswire; 2015 [cited 2023 Sep 10]. Available from: https://www.globenewswire.com/news-release/2015/04/15/724774/26312/en/Versatile-Point-of-Care-Ultrasound-Technology-Quickly-Gaining-Market-Share-in-the-UnitedStates.html.
20. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest 2009; 135:1050-60.
21. Via G, Hussain A, Wells M, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014; 27:683.
22. Laursen CB, Sloth E, Lambrechtsen J, et al. Focused sonography of the heart, lungs, and deep veins identifies missed lifethreatening conditions in admitted patients with acute respiratory symptoms. Chest 2013; 144:1868-75.
23. Kovell LC, Ali MT, Hays AG, et al. Defining the role of pointof-care ultrasound in cardiovascular disease. Am J Cardiol 2018; 122:1443-50.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients: Part II: cardiac ultrasonography. Crit Care Med 2016; 44:1206-27.
25. Marbach JA, Almufleh A, Di Santo P, et al. Comparative accuracy of focused cardiac ultrasonography and clinical examination for left ventricular dysfunction and valvular heart disease: a systematic review and meta-analysis. Ann Intern Med 2019; 171:264-72.
26. Singh Y, Tissot C, Fraga MV, et al. International evidence-based guidelines on point of care ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 2020; 24:65.
27. Zanobetti M, Poggioni C, Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest 2011; 139:1140-7.
28. Laursen CB, Sloth E, Lassen AT, et al. Point-of-care ultrasonography in patients admitted with respiratory symptoms: a single-blind, andomized controlled trial. Lancet Respir Med 2014; 2:638-46.
29. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134:117-25.
30. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest 2012; 141:703-8.
31. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med 2014; 21:843-52.
32. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res 2014; 15:50.
34. Blaivas M, Harwood RA, Lambert MJ. Decreasing length of stay with emergency ultrasound examination of the gallbladder. Acad Emerg Med 1999; 6:1020-3.
35. Durston W, Carl ML, Guerra W, et al. Comparison of quality and cost-effectiveness in the evaluation of symptomatic cholelithiasis with different approaches to ultrasound availability in the ED. Am J Emerg Med 2001; 19:260-9.
36. Miller AH, Pepe PE, Brockman CR, Delaney KA. ED ultrasound in hepatobiliary disease. J Emerg Med 2006; 30:69-74.
37. Young N, Kinsella S, Raio CC, et al. Economic impact of additional radiographic studies after registered diagnostic medical sonographer (RDMS)-certified emergency physician-performed identification of cholecystitis by ultrasound. J Emerg Med 2010; 38:645-51.
38. Ross M, Brown M, McLaughlin K, et al. Emergency physicianperformed ultrasound to diagnose cholelithiasis: a systematic review. Acad Emerg Med 2011; 18:227-35.
39. Kendall JL, Shimp RJ. Performance and interpretation of focused right upper quadrant ultrasound by emergency physicians. J Emerg Med 2001; 21:7-13.
40. Blaivas M, Adhikari S. Diagnostic utility of cholescintigraphy in emergency department patients with suspected acute cholecystitis: comparison with bedside RUQ ultrasonography. J Emerg Med 2007; 33:47-52.
41. Burge HJ, Middleton WD, McClennan BL, Hildebolt CF. Ureteral jets in healthy subjects and in patients with unilateral ureteral calculi: comparison with color Doppler US. Radiology 1991; 180:437-42.
42. Strehlau J, Winkler P, de la Roche J. The uretero-vesical jet as a functional diagnostic tool in childhood hydronephrosis. Pediatr Nephrol 1997; 11:460-7.
43. Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991; 13:452-8.
44. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med 2013; 20:128-38.
45. Schmutz GR, Benko A, Fournier L, Peron JM, Morel E, Chiche L. Small bowel obstruction: role and contribution of sonography. Eur Radiol 1997; 7:1054-8.
46. Hefny AF, Corr P, Abu-Zidan FM. The role of ultrasound in the management of intestinal obstruction. J Emerg Trauma Shock 2012; 5:84-6.
47. Sargar KM, Siegel MJ. Sonography of acute appendicitis and its mimics in children. Indian J Radiol Imaging 2014; 24:163-70.
48. Nicola R, Dogra V. Ultrasound: the triage tool in the emergency department: using ultrasound first. Br J Radiol 2016; 89:20150790.
49. American College of Emergency Physicians. Emergency ultrasound imaging criteria compendium. American College of Emergency Physicians. Ann Emerg Med 2006; 48:487-510.
50. Oulego-Erroz I, Gonzalez-Cortes R, Garcia-Soler P, et al. Ultrasound-guided or landmark techniques for central venous catheter placement in critically ill children. Intensive Care Med 2018; 44:61-72.
51. Young B, Onwochei D, Desai N. Conventional landmark palpation vs. preprocedural ultrasound for neuraxial analgesia and anaesthesia in obstetrics: a systematic review and meta-analysis with trial sequential analyses. Anaesthesia 2021; 76:818-31.
52. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided atheterization of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care 2006; 10:R162.
53. Wu T, Dong Y, Song Hx, Fu Y, Li JH. Ultrasound-guided versus landmark in knee arthrocentesis: a systematic review. Semin Arthritis Rheum 2016; 45:627-32.
54. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev 2015; 1:CD006962.
55. Lv Y, Liu H, Yu P, et al. Evaluating the long-, short-, and oblique-axis approaches for ultrasound-guided vascular access cannulation. J Ultrasound Med 2019; 38:347-55.
56. Ye W, Li D, Ji X, et al. Real-time ultrasound-guided internal jugular vein cannulation by oblique-axis in-plane: practice at the Fourth Hospital of Hebei Medical University. Int J Clin Pract 2021; 75:e13673.
57. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth 2016; 116:370-6.
58. Arora NR, Maddali MM, Al-Sheheimi RA, Al-Mughairi H, Panchatcharam SM. Ultrasound-guided out-of-plane versus in-plane radial artery cannulation in adult cardiac surgical patients. J Cardiothorac Vasc Anesth 2021; 35:84-8.
59. Gold AK, Al-Ghofaily L, Wenger IE, Augoustides JG. Arterial access-choosing in-plane or out-of-plane imaging for vessel cannulation. J Cardiothorac Vasc Anesth 2021; 35:89-90.
60. Forneris G, Marciello A, Savio D, Gallieni M. Ultrasound in central venous access for hemodialysis. J Vasc Access 2021; 22(1_suppl):97-105.
61. Montrief T, Ramzy M, Long B. Micropuncture kits for difficult vascular access. Am J Emerg Med 2021; 46:651-2.
62. Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982; 57:769-74.
63. Lau VI, Jaidka A, Wiskar K, et al. Better with ultrasound: transcranial Doppler. Chest 2020; 157:142-50.
64. Montrief T, Alerhand S, Jewell C, Scott J. Incorporation of transcranial Doppler into the ED for the neurocritical care patient. Am J Emerg Med 2019; 37:1144-52.
65. Maurer M, Shambal S, Berg D, et al. Differentiation between intracerebral hemorrhage and ischemic stroke by transcranial color-coded duplex-sonography. Stroke 1998; 29:2563-7.
66. Meyer-Wiethe K, Sallustio F, Kern R. Diagnosis of intracerebral hemorrhage with transcranial ultrasound. Cerebrovasc Dis 2009; 27 Suppl 2:40-7.
67. Avila-Reyes D, Acevedo-Cardona AO, Gomez-Gonzalez JF, Echeverry-Piedrahita DR, Aguirre-Florez M, Giraldo-Diaconeasa A. Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): narrative review article. Ultrasound J 2021; 13:46.
68. Oh J, Cha KC, Lee JH, et al. 2020 Korean guidelines for cardiopulmonary resuscitation. Part 4. Adult advanced life support. Clin Exp Emerg Med 2021; 8(S):S26-40.
69. Panchal AR, Bartos JA, Cabanas JG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020; 142(16_suppl_2):S366-468.
70. Grmec S. Comparison of three different methods to confirm tracheal tube placement in emergency intubation. Intensive Care Med 2002; 28:701-4.
71. Takeda T, Tanigawa K, Tanaka H, Hayashi Y, Goto E, Tanaka K. The assessment of three methods to verify tracheal tube placement in the emergency setting. Resuscitation 2003; 56:153-7.
72. Tanigawa K, Takeda T, Goto E, Tanaka K. Accuracy and reliability of the self-inflating bulb to verify tracheal intubation in out-of-hospital cardiac arrest patients. Anesthesiology 2000; 93:1432-6.
73. Milling TJ, Jones M, Khan T, et al. Transtracheal 2-d ultrasound for identification of esophageal intubation. J Emerg Med 2007; 32:409-14.
74. Werner SL, Smith CE, Goldstein JR, Jones RA, Cydulka RK. Pilot study to evaluate the accuracy of ultrasonography in confirming endotracheal tube placement. Ann Emerg Med 2007; 49:75-80.
75. Chou HC, Tseng WP, Wang CH, et al. Tracheal rapid ultrasound exam (T.R.U.E.) for confirming endotracheal tube placement during emergency intubation. Resuscitation 2011; 82:1279-84.
76. Kabil AE, Ewis AM, Al-Ashkar AM, Abdelatif MA, Nour MO. Real-time tracheal ultrasonography for confirming endotracheal tube placement. Egypt J Bronchol 2018; 12:323-8.
77. Chai HS, Kim YM, Park GJ, et al. Comparison between internal jugular vein access using midline catheter and peripheral intravenous access during cardiopulmonary resuscitation in adults. SAGE Open Med 2023; 11:20503121231175318.
78. Park JB, Jo YI, Cho YS, et al. Factors affecting cardiopulmonary resuscitation hands-off time in an emergency room. J Korean Soc Emerg Med 2012; 23:221-8.
79. Kasatkin A, Urakov A, Nigmatullina A. Ultrasound-guided vascular access during cardiopulmonary resuscitation. In: Aslanidis TK, editor. Special topics in resuscitation. IntechOpen; 2018. p. 41-55.
80. Lazaar S, Mazaud A, Delsuc C, et al. Ultrasound guidance for urgent arterial and venous catheterisation: randomised controlled study. Br J Anaesth 2021; 127:871-8.
81. Dronen S, Thompson B, Nowak R, Tomlanovich M. Subclavian vein catheterization during cardiopulmonary resuscitation: a prospective comparison of the supraclavicular and infraclavicular percutaneous approaches. JAMA 1982; 247:3227-30.
82. Mallin M, Louis H, Madsen T. A novel technique for ultrasound-guided supraclavicular subclavian cannulation. Am J Emerg Med 2010; 28:966-9.
83. Breitkreutz R, Price S, Steiger HV, et al. Focused echocardiographic evaluation in life support and peri-resuscitation of emergency patients: a prospective trial. Resuscitation 2010; 81:1527-33.
84. Gaspari R, Weekes A, Adhikari S, et al. Emergency department point-of-care ultrasound in out-of-hospital and in-ED cardiac arrest. Resuscitation 2016; 109:33-9.
85. Jensen MB, Sloth E, Larsen KM, Schmidt MB. Transthoracic echocardiography for cardiopulmonary monitoring in intensive care. Eur J Anaesthesiol 2004; 21:700-7.
86. Breitkreutz R, Walcher F, Seeger FH. Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med 2007; 35(5 Suppl):S150-61.
87. Hernandez C, Shuler K, Hannan H, Sonyika C, Likourezos A, Marshall J. C.A.U.S.E.: cardiac arrest ultra-sound exam: a better approach to managing patients in primary non-arrhythmogenic cardiac arrest. Resuscitation 2008; 76:198-206.
88. Lichtenstein D, Malbrain ML. Critical care ultrasound in cardiac arrest: technological requirements for performing the SESAME-protocol: a holistic approach. Anaesthesiol Intensive Ther 2015; 47:471-81.
89. Atkinson P, Bowra J, Milne J, et al. International Federation for Emergency Medicine consensus statement: sonography in hypotension and cardiac arrest (SHoC): an international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest. CJEM 2017; 19:459-70.
90. Flato UA, Paiva EF, Carballo MT, Buehler AM, Marco R, Timerman A. Echocardiography for prognostication during the resuscitation of intensive care unit patients with non-shockable rhythm cardiac arrest. Resuscitation 2015; 92:1-6.
91. Hwang SO, Zhao PG, Choi HJ, et al. Compression of the left ventricular outflow tract during cardiopulmonary resuscitation. Acad Emerg Med 2009; 16:928-33.
92. Zanatta M, Lorenzi C, Scorpiniti M, Cianci V, Pasini R, Barchitta A. Ultrasound-guided chest compressions in out-ofhospital cardiac arrests. J Emerg Med 2020; 59:e225-33.
93. Shoenberger JM, Massopust K, Henderson SO. The use of bedside ultrasound in cardiac arrest. Cal J Emerg Med 2007; 8:47-50.
94. Vallet B, Robin E, Lebuffe G. Resuscitation from circulatory shock. In: Vincent JL, Abraham E, Moore FA, Kochanek PM, Fink MP, editors. Textbook of critical care. 7th ed. Elsevier; 2017. p. 623-7.
95. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am 2010; 28:29-56.
96. Seif D, Perera P, Mailhot T, Riley D, Mandavia D. Bedside ultrasound in resuscitation and the rapid ultrasound in shock protocol. Crit Care Res Pract 2012; 2012:503254.
97. Atkinson PR, McAuley DJ, Kendall RJ, et al. Abdominal and Cardiac Evaluation with Sonography in Shock (ACES): an approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg Med J 2009; 26:87-91.
98. Atkinson P, Bowra J, Milne J, et al. International Federation for Emergency Medicine Consensus Statement: sonography in hypotension and cardiac arrest (SHoC): an international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest: corrigendum. CJEM 2017; 19:327.
99. Kirkpatrick AW, Sirois M, Laupland KB, et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the Extended Focused Assessment with Sonography for Trauma (EFAST). J Trauma 2004; 57:288-95.
100. Dulchavsky SA, Henry SE, Moed BR, et al. Advanced ultrasonic diagnosis of extremity trauma: the FASTER examination. J Trauma 2002; 53:28-32.
101. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38:577-91.
102. Mennicke M, Gulati K, Oliva I, et al. Anatomical distribution of traumatic pneumothoraces on chest computed tomography: implications for ultrasound screening in the ED. Am J Emerg Med 2012; 30:1025-31.
103. Elliott DS, Baker PA, Scott MR, Birch CW, Thompson JM. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia 2010; 65:889-94.
104. Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med 2010; 17:11-7.
105. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med 1999; 25:955-8.
106. Ferrada P, Murthi S, Anand RJ, Bochicchio GV, Scalea T. Transthoracic focused rapid echocardiographic examination: realtime evaluation of fluid status in critically ill trauma patients. J Trauma 2011; 70:56-64.
107. Gunst M, Ghaemmaghami V, Sperry J, et al. Accuracy of cardiac function and volume status estimates using the bedside echocardiographic assessment in trauma/critical care. J Trauma 2008; 65:509-16.
108. Natarajan B, Gupta PK, Cemaj S, Sorensen M, Hatzoudis GI, Forse RA. FAST scan: is it worth doing in hemodynamically stable blunt trauma patients? Surgery 2010; 148:695-701.
109. Miller DL, Abo A, Abramowicz JS, et al. Diagnostic ultrasound safety review for point-of-care ultrasound practitioners. J Ultrasound Med 2020; 39:1069-84.
110. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a metaanalysis. Pediatrics 2015; 135:714-22.
111. Longjohn M, Wan J, Joshi V, Pershad J. Point-of-care echocardiography by pediatric emergency physicians. Pediatr Emerg Care 2011; 27:693-6.
112. Riera A, Hsiao AL, Langhan ML, Goodman TR, Chen L. Diagnosis of intussusception by physician novice sonographers in the emergency department. Ann Emerg Med 2012; 60:264-8.
113. Friedman N, Pancer Z, Savic R, et al. Accuracy of point-ofcare ultrasound by pediatric emergency physicians for testicular torsion. J Pediatr Urol 2019; 15:608.
114. Holmes JF, Kelley KM, Wootton-Gorges SL, et al. Effect of abdominal ultrasound on clinical care, outcomes, and resource use among children with blunt torso trauma: a randomized clinical trial. JAMA 2017; 317:2290-6.
115. Pai DR, Thapa M. Musculoskeletal ultrasound of the upper extremity in children. Pediatr Radiol 2013; 43 Suppl 1:S48-54.
116. de Souza TH, Brandao MB, Nadal JA, Nogueira RJ. Ultrasound guidance for pediatric central venous catheterization: a meta-analysis. Pediatrics 2018; 142:e20181719.
117. Fraga MV, Stoller JZ, Glau CL, et al. Seeing is believing: ultrasound in pediatric procedural performance. Pediatrics 2019; 144:e20191401.
118. Thapa M, Vo JN, Shiels WE 2nd. Ultrasound-guided musculoskeletal procedures in children. Pediatr Radiol 2013; 43 Suppl 1:S55-60.
119. Golea AC. Is point of care ultrasound (PoCUS) integrated in clinical exam a key-tool for the future emergency physician? Med Ultrason 2017; 19:250-1.
120. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med 2009; 53:550-70.
121. Gardner KF, Clattenburg EJ, Wroe P, Singh A, Mantuani D, Nagdev A. The Cardiac Arrest Sonographic Assessment (CASA) exam: a standardized approach to the use of ultrasound in PEA. Am J Emerg Med 2018; 36:729-31.
122. Zanobetti M, Coppa A, Nazerian P, et al. Chest abdominal-focused assessment sonography for trauma during the primary survey in the emergency department: the CA-FAST protocol. Eur J Trauma Emerg Surg 2018; 44:805-10.
123. Leroux P, Javaudin F, Le Bastard Q, et al. Goal-directed ultrasound protocol in patients with nontraumatic undifferentiated shock in the emergency department: prospective dual centre study. Eur J Emerg Med 2021; 28:306-11.
124. Ramadan A, Abdallah T, Abdelsalam H, Mokhtar A, Razek AA. Accuracy of echocardiography and ultrasound protocol to identify shock etiology in emergency department. BMC Emerg Med 2022; 22:117.
125. Gallard E, Redonnet JP, Bourcier JE, et al. Diagnostic performance of cardiopulmonary ultrasound performed by the emergency physician in the management of acute dyspnea. Am J Emerg Med 2015; 33:352-8.
126. Vauthier C, Chabannon M, Markarian T, et al. Point-of-care chest ultrasound to diagnose acute heart failure in emergency department patients with acute dyspnea: diagnostic performance of an ultrasound-based algorithm. Emergencias 2021; 33:441-6.
127. Ahn JH, Jeon J, Toh HC, et al. SEARCH 8Es: a novel point of care ultrasound protocol for patients with chest pain, dyspnea or symptomatic hypotension in the emergency department. PLoS One 2017; 12:e0174581.
128. Atar S, Feldman A, Darawshe A, Siegel RJ, Rosenfeld T. Utility and diagnostic accuracy of hand-carried ultrasound for emergency room evaluation of chest pain. Am J Cardiol 2004; 94:408-9.
129. Colony MD, Edwards F, Kellogg D. Ultrasound assisted evaluation of chest pain in the emergency department. Am J Emerg Med 2018; 36:533-9.
130. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2013; 26:567-81.
131. Pellikka PA. Cardiac point-of-care ultrasound (POCUS): extending the reach. J Am Soc Echocardiogr 2023; 36:263-4.
132. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients: part I: general ultrasonography. Crit Care Med 2015; 43:2479-502.
133. Atkinson P, Bowra J, Lambert M, Lamprecht H, Noble V, Jarman B. International Federation for Emergency Medicine point of care ultrasound curriculum. CJEM 2015; 17:161-70.
134. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med 2014; 89:1681-6.
135. Russell FM, Zakeri B, Herbert A, Ferre RM, Leiser A, Wallach PM. The state of point-of-care ultrasound training in undergraduate medical education: findings from a national survey. Acad Med 2022; 97:723-7.
Fig. 1.Three types of ultrasound devices for point-of-care ultrasound. (A) Compact cart-based. (B) Hand-carried. (C) Handheld or pocket-sized. 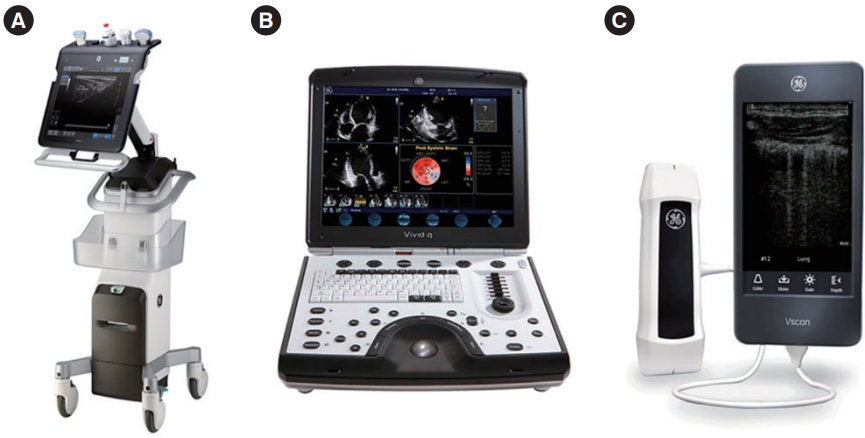
Fig. 3.Various lung artifacts. (A) The A-line artifacts are created by reverberation of ultrasound waves at the pleural line. (B) The B-line artifacts are hyperechoic vertical artifacts which extend from the pleural line to the bottom of the image. (C) If consolidation touches the pleural line, ultrasound penetrates it directly, resulting in visualization without artifacts. Each arrow points to the corresponding artifact finding. 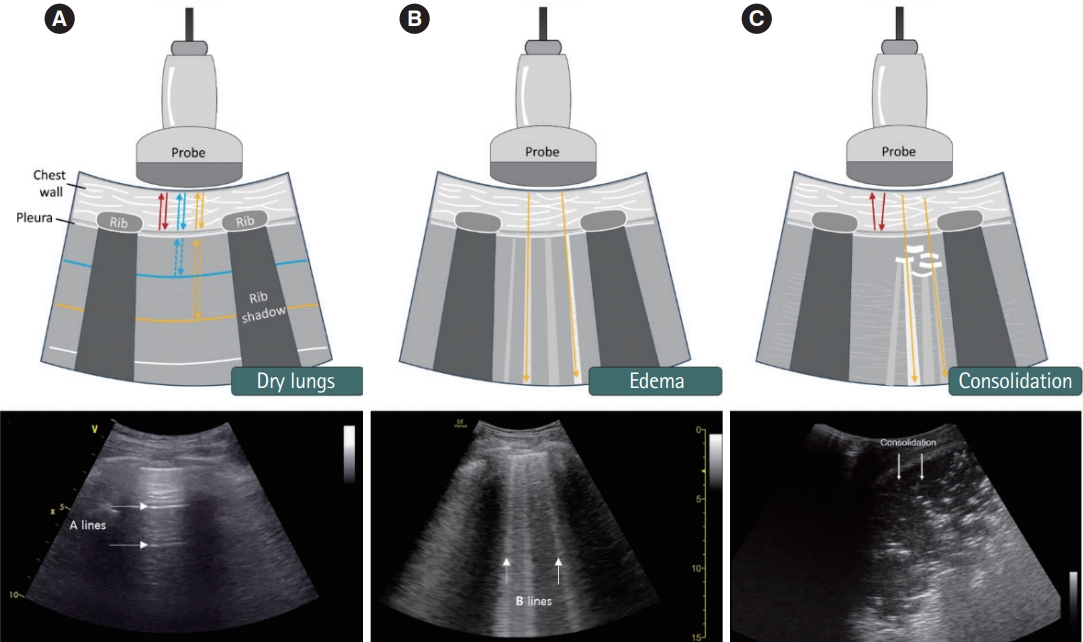
Fig. 4.M-mode ultrasound images . (A) Normal lung. The sea-shore sign in M-mode ultrasound. P indicates the pleural line. (B) Pneumothorax. The stratosphere or barcode sign in which there is no movement below the pleural line. 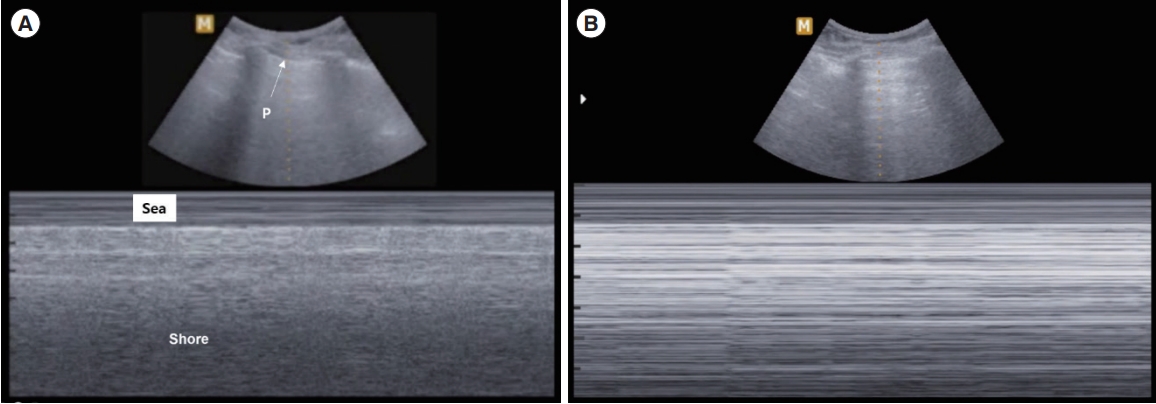
Fig. 5.Acute hematoma. (A) Computed tomography shows left intracranial hemorrhage with intraventricular hemorrhage. (B) Transcranial ultrasound via right temporal view shows an echogenic mass on the bottom of the screen (arrows), which is identified as intracranial hemorrhage on the same patient. 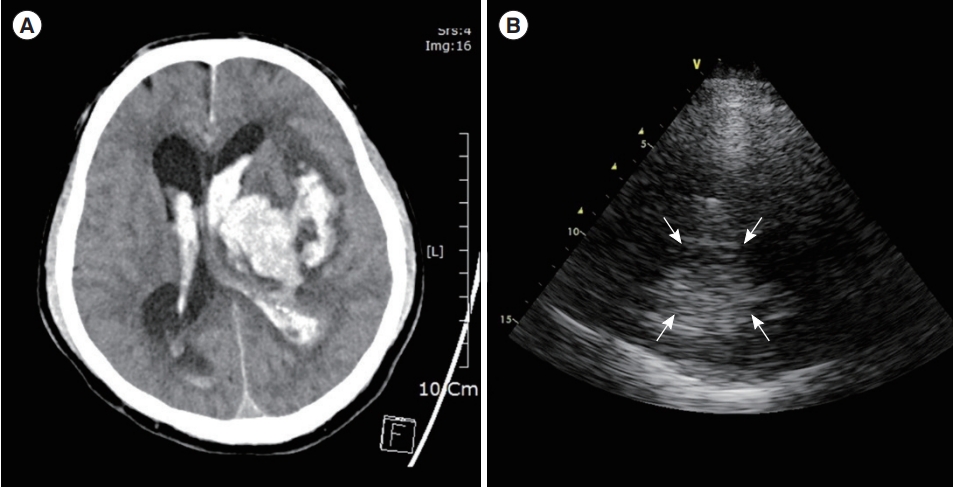
Fig. 6.Midline shifting with acute hematoma. (A) Computed tomography shows shifted midline compressed by left subdural hematoma. (B) Displaced third ventricle (arrows) with acute hematoma (asterisk) is shown on the transcranial ultrasonography with the right temporal approach. 
Fig. 7.Stepwise evaluation using the RUSH (Rapid Ultrasound in Shock) protocol to diagnose the category of shock. 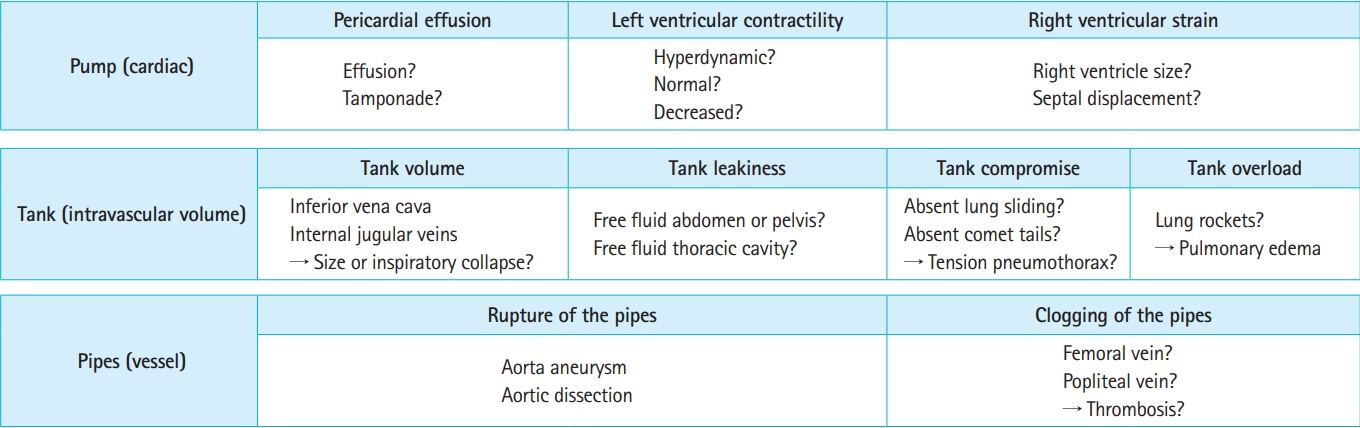
Fig. 11.Critical versus noncritical point-of-care ultrasound (POCUS) applications in the emergency department. 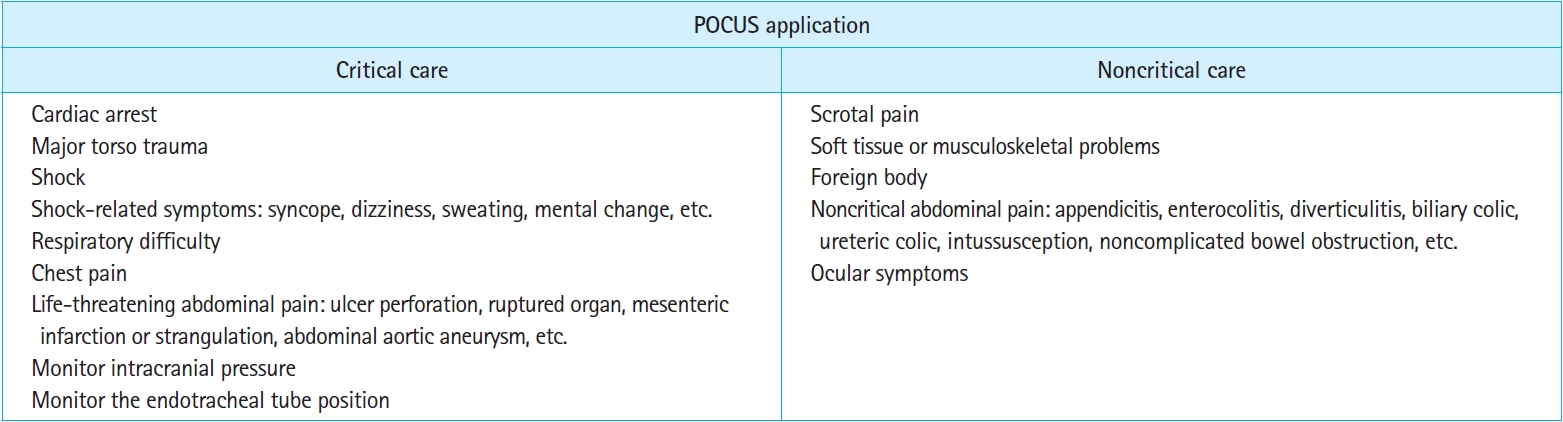
Table 1.Clinical scenarios that require focused cardiac ultrasound and what can be identified through assessment Table 2.The key ultrasonography finding for evaluating the category and causes of undifferentiated shock |
|
||||||||||||||||||||||||||||||||||||||