Diagnostic performance and optimal cut-off values of cardiac biomarkers for predicting cardiac injury in carbon monoxide poisoning
Article information
Abstract
Objective
This study aimed to compare the diagnostic performance of cardiac biomarkers and to evaluate the optimal cut-off values for echocardiographic cardiac injury prediction in patients with carbon monoxide (CO) poisoning.
Methods
This retrospective observational cohort study included adult patients with acute CO poisoning. Patients who did not undergo transthoracic echocardiography, which was used to define patients with cardiac injury (ejection fraction <55%), were excluded. The area under the curve was used to evaluate diagnostic performance for cardiac injury prediction. Mann-Whitney U, chi-square, and Fisher exact tests were used to analyze data.
Results
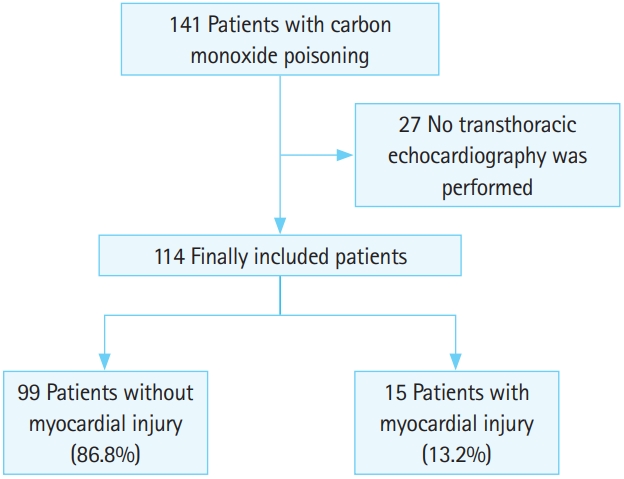
After excluding the 27 patients who did not undergo echocardiography, 114 patients were included in the study. Fifteen (13.2%) patients had cardiac injury. The area under the curve values for the B-type natriuretic peptide, creatine kinase-myocardial band, and troponin I were 0.711 (95% confidence interval [CI], 0.527–0.895; P=0.011), 0.766 (95% CI, 0.607–0.926; P=0.001), and 0.801 (95% CI, 0.647–0.955; P<0.001), respectively, with optimal cut-off values of 330 pg/mL, 10.1 ng/mL, and 0.455 ng/mL, respectively. The sensitivity, specificity, and positive and negative predictive values of troponin I were 67%, 91%, 53%, and 95%, respectively.
Conclusion
Troponin I showed the best diagnostic performance for predicting cardiac injury in patients with CO poisoning. A cut-off value of 0.455 ng/mL appeared optimal for cardiac injury prediction. However, further studies on cardiac biomarkers and other diagnostic tools in CO poisoning are needed given the low sensitivity of troponin I.
INTRODUCTION
Carbon monoxide (CO), generated from the incomplete combustion of carbon fuels, can be found everywhere. For example, installed gas devices, heaters, generators, and vehicle exhaust are sources of CO. Because it is colorless, odorless, and non-irritating, it is not easily recognized, and its impact is often underestimated. CO poisoning is very common in the United States and is responsible for approximately 50,000 emergency room visits and 1,500 deaths yearly [1,2]. The morbidity of CO poisoning is attributed to delayed neuropsychologic sequelae, often accompanied by cognitive dysfunction, memory impairment, language disturbance, emotional instability, and akinetic mutism [3,4]. Therefore, to date, delayed neuropsychologic sequelae have been the focus of research into CO poisoning.
In the acute phase, CO poisoning can cause dysfunction of various organs, especially the brain, heart, kidney, nerve, and muscle. Cardiac injury, a major complication in patients with CO poisoning, is reported in approximately 33% of patients with moderateto-severe CO poisoning [5]. Angina, arrhythmia, myocardial infarction, and cardiac arrest have been reported as cardiac complications of CO poisoning [6-10]. The mortality of patients with CO poisoning and cardiac injury is 1.9-fold higher than that of the general population [5,11]. Additionally, long-term (7–8 years) follow-up of patients with CO poisoning who suffered acute phase cardiac injury revealed that their rates of heart disease-induced complications and mortality were 2 to 3 times higher than those of patients without cardiac injury [12,13].
Therefore, early recognition of cardiac injury in patients with CO poisoning is important for the prediction of mortality and morbidity or the decision to perform further management, such as hyperbaric oxygen treatment. Various tests, such as echocardiography, cardiac computed tomography, cardiac magnetic resonance imaging, and myocardial single photon emission computed tomography, can be used to diagnose cardiac injury; however, only a few hospitals can perform these tests, especially in the emergency department (ED). Thus, it may be useful to use cardiac biomarkers as an alternative to these special tests. However, no study has compared the diagnostic performance and optimal cut-off value of cardiac biomarkers for cardiac injury prediction in CO poisoning. Therefore, we aimed to compare the diagnostic performance of cardiac biomarkers and evaluate the optimal cut-off values for echocardiographic cardiac injury prediction in patients with CO poisoning.
METHODS
Study design and setting
A retrospective observational cohort study was conducted at a single center, the Hanyang University Hospital, Seoul, Korea. Before commencement of this study, the hospital’s institutional review board approved this review of patient data (HYUH 2019-04- 053-003) and waived the requirement for informed patient consent owing to the retrospective nature of the study. Adult patients (≥18 years of age) with acute CO poisoning who presented at the ED were included in the study, which was conducted from September 2018 to March 2019. CO poisoning was diagnosed by the emergency physician, based on clinical history with a suspected CO source, CO poisoning-associated clinical symptoms or signs, and Carboxyhemoglobin (COHb) levels. CO poisoning diagnostic criteria were ≥5% for smokers, ≥3% for non-smokers, and ≥5% when current smoking status was unknown [14]. The COHb level could be below this threshold if patients had visited the ED a long time after the end of CO exposure. In such cases, the patients were enrolled based on their clinical history or by considering the half-life of COHb. Patients who did not undergo transthoracic echocardiography were excluded. Cardiac injury was defined if echocardiography revealed that the left ventricular ejection fraction was <55% [15].
Data collection and study variables
Predefined variables were recorded for patients by using CO poisoning templates in the hospital. For patients transferred from another hospital, the initial COHb levels at the first hospital visit and our hospital were recorded. The time of the initial CO exposure and the duration of exposure were recorded based on the patient’s or guardian’s statement. Blood tests and echocardiography were conducted for all patients; using the same machine (HS50; Samsung Medicine, Seoul, Korea), the emergency physician conducted transthoracic echocardiography on the first day of ED presentation. Parasternal long axis, short axis, and apical 4-and 2-chamber views were obtained in accordance with the American Society of Echocardiography guidelines [15]. The left ventricular ejection fraction was assessed by visual estimation. Echocardiography was conducted under the supervision of a board-certified emergency physician to ensure diagnostic accuracy. Thereafter, echocardiography was reassessed by a cardiologist specialized in echocardiography (RH), who made the final decision on any inconsistencies. From the day of hospitalization to discharge, blood tests were conducted for troponin I, B-type natriuretic peptide (BNP), creatine kinase-myocardial band (CK-MB), and creatine kinase (CK). The normal ranges of these biomarkers are as follows: troponin I <0.04 ng/mL; BNP <100 pg/mL; CK-MB 0.3–4 ng/mL; and CK 40–200 U/L.
Treatment protocol
A non-rebreather mask with 100% oxygen was applied to all patients with CO poisoning until hyperbaric oxygen treatment was performed or the patient was discharged without hyperbaric oxygen treatment. The indications for hyperbaric oxygen treatment are as follows: first, when the initial arterial blood CO-hemoglobin concentration was, or was assumed to be, considering its half-life, ≥ 25%; second, when there were neurological symptoms, such as loss of consciousness, coma, confusion, focal neurologic deficit, or seizure; third, when there was evidence of myocardial ischemia or end-organ ischemia; and fourth, when the CO concentration measured in a pregnant woman was ≥ 15%. Three sessions of hyperbaric oxygen treatment were routinely provided, and an additional session was provided if diffusion-weighted brain magnetic resonance imaging showed evidence of brain injury or echocardiographic findings/cardiac biomarkers were suggestive of cardiac injury. As specified by the hospital protocol, hyperbaric oxygen therapy was performed for 2 hours. The pressure was increased over 30 minutes to 2.5 absolute pressures (atmosphere absolute), maintained at 2.5 atmosphere absolute for 60 minutes, and then lowered over 30 minutes. As our hospital uses a monoplace chamber without medical staff and proper monitoring devices, intubated or hemodynamically unstable patients did not undergo hyperbaric oxygen treatment.
Statistical analysis
All continuous variables are presented as mean with standard deviation or median with interquartile range, according to their distribution. Categorical variables are expressed as frequency and percentage. Continuous variables were compared by using the Mann-Whitney U-test, whereas categorical variables were analyzed by using the chi-square test and Fisher exact test. The area under the curve (AUC) was calculated to compare the diagnostic performance of cardiac biomarker levels. The optimal cut-off values to maximize sensitivity and specificity were determined by using the Youden index. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), with the cut-off value of each cardiac biomarker were calculated. All reported Pvalues were 2-sided, and P-values <0.05 were considered statistically significant. All statistical analyses were performed by using PASW Statistics ver. 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
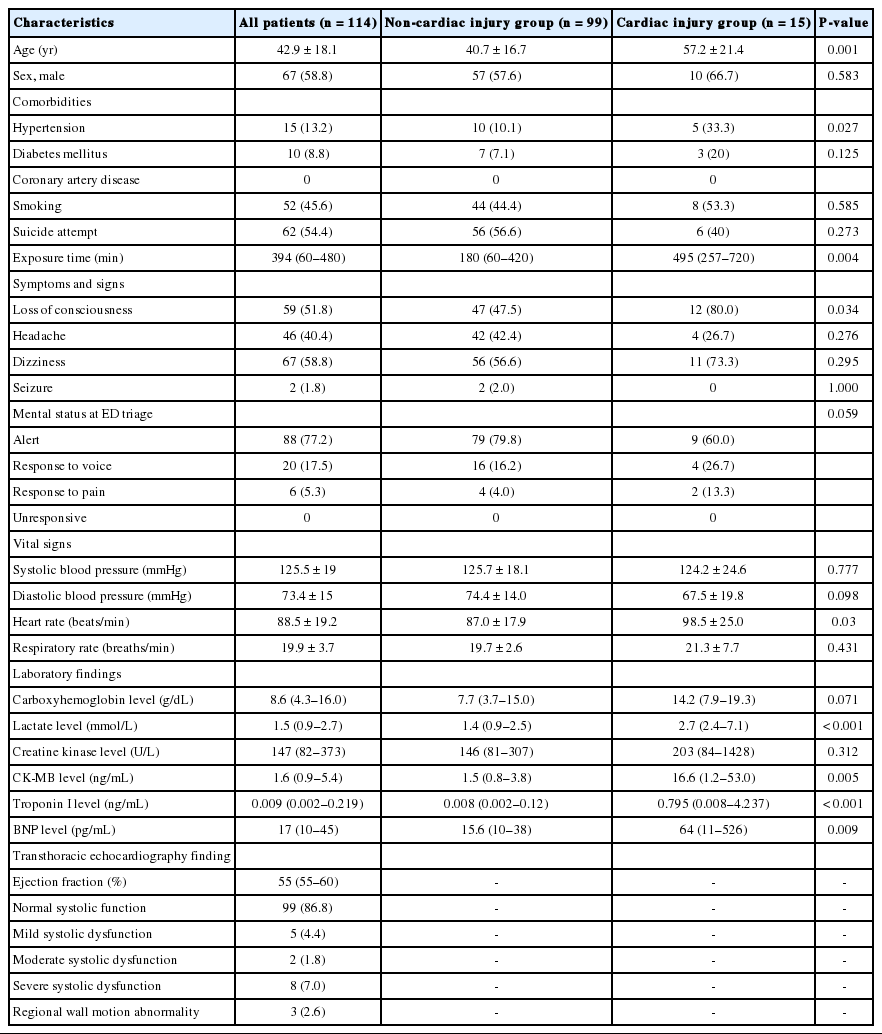
Of the 141 patients with CO poisoning who visited the hospital during the study period, 114 were included, as 27 did not undergo echocardiography (Fig. 1). As shown in Table 1, the mean age of the cohort was 42.9 years, and 67 (58.8%) patients were male. Fifty two (45.6%) patients were smokers, 15 (13.2%) had hypertension, and 10 (8.8%) had diabetes. The median CO exposure time was 394 minutes, and the most common symptoms/signs were dizziness (n=67, 58.8%), loss of consciousness (n=59), headache (n=46), and seizure (n=2). Mental status at the scene was alert for 66 (57.9%) patients. The median COHb concentration was 8.6 g/dL, and the median troponin I concentration was 0.009 ng/mL. Fifteen (13.2%) patients had cardiac injury, which was defined as ejection fraction <55% based on echocardiography on the hospital day 1. Mild systolic dysfunction was observed in 5 (4.4%) patients. Typical mid-apical regional wall motion abnormalities with hyperactive contractility of the basal left ventricular segments was observed in 2 (1.8%) patients.
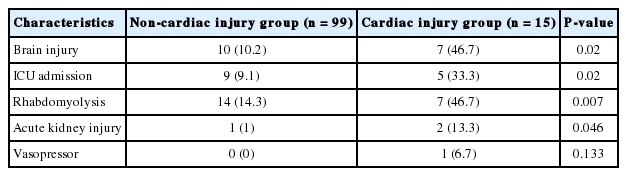
As shown in Table 1, the mean age of patients in the non-cardiac injury group was significantly younger than that of those in the cardiac injury group (40.7 vs. 57.2 years old, P=0.001). A significantly higher number of patients had hypertension in the cardiac injury group than in the non-cardiac injury group (33.3% vs. 10.1%, P=0.027). There was no significant difference in the proportions of patients with diabetes and smokers. Loss of consciousness was more prevalent in the cardiac injury group than in the non-cardiac injury group (80% vs. 47.5%, P=0.034). The mean initial heart rate was significantly higher in the cardiac injury group than in the non-cardiac injury group (98.5 vs. 87.0 beats/min, P=0.03). The median COHb concentration was higher in the cardiac injury group than in the non-cardiac injury group, although the difference was not significant (14.2 and 7.7 g/dL, respectively; P=0.071). The median CK-MB concentration was significantly higher in the cardiac injury group than in the noncardiac injury group (16.6 vs. 1.5 ng/mL, P=0.005). The proportions of patients with brain injury, intensive care unit admission, rhabdomyolysis, and acute kidney injury were significantly higher in the cardiac injury group than in the non-cardiac injury group (Table 2). There was no difference between the two groups in the proportion of vasopressor use. No in-hospital mortality was observed for the whole study population.
The diagnostic performance of cardiac biomarkers for predicting cardiac injury in patients with CO poisoning was compared. The AUC values of BNP, CK-MB, and troponin I were 0.711 (95% confidence interval [CI], 0.647–0.955; P<0.001), 0.766 (95% CI, 0.607–0.926; P=0.001), and 0.801 (95% CI, 0.647–0.955; P<0.001), respectively (Fig. 2). Additionally, the optimal cut-off value maximizing the sum of sensitivity and specificity was determined. CK-MB showed 60% sensitivity, 91% specificity, 50% PPV, and 94% NPV with a cut-off value of ≥10.1 ng/mL for cardiac injury prediction (Table 3). A cut-off value of ≥330 pg/mL was observed for BNP, with 43% sensitivity, 100% specificity, 100% PPV, and 92% NPV, and a cut-off value of ≥0.455 ng/mL was observed for troponin I, with 67% sensitivity, 91% specificity, 53% PPV, and 95% NPV.
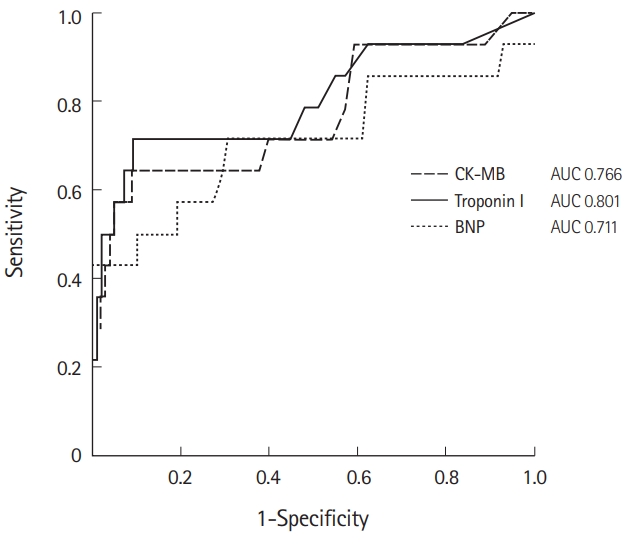
Receiver operating characteristic curves of creatine kinase-myocardial band (CK-MB), B-type natriuretic peptide (BNP), and troponin I for predicting cardiac injury in carbon monoxide poisoning. AUC, area under the curve.
DISCUSSION
Echocardiography-defined cardiac injury occurred in 13.2% of patients with CO poisoning. Among the cardiac biomarkers, troponin I showed the best diagnostic performance for predicting cardiac injury in patients with CO poisoning. The optimal cut-off value of troponin I was 0.455 ng/mL. To the best of our knowledge, this is the first study to investigate the diagnostic performance and optimal cut-off value of cardiac biomarkers for predicting cardiac injury in CO poisoning. The results of this study may help physicians to determine whether a patient has a cardiac injury, especially in the absence of echocardiography. It can also help with decisions regarding patient transfer for hyperbaric oxygen therapy. Transthoracic echocardiography was performed in 80% of cases, and the diagnostic accuracy was enhanced by the interpretation of a cardiologist. All echocardiography and blood tests were performed on the first day of presentation to the ED to minimize the time between the two diagnostic tools.
CO induced cardiac injury is associated with both short-term and long-term morbidity and mortality. Shen et al. [11] reported that it was associated with in-hospital mortality and neurologic sequelae. Cardiac injury reportedly occurred frequently in patients hospitalized for moderate-to-severe CO poisoning and was a significant predictor of mortality [5]. In this study, CK-MB levels in the absence of echocardiography revealed that cardiac injury occurred in 37% of patients. Kalay et al. [6] reported that 40% of patients with CO poisoning showed decreased left ventricular ejection fraction (<45%), whereas we observed a lower proportion of patients with decreased ejection fraction. Cha et al. [16] observed abnormal echocardiographic findings in 40.6% of patients, which defined normal systolic function as an ejection fraction >50% (different from our definition). Compared with our study, the patients in their study appeared to have a higher severity in terms of the Glasgow Coma Scale score and initial troponin I concentration, suggesting that the number of patients exhibiting abnormal systolic function was relatively low in our study. However, a comparison of the cardiac biomarker cut-off values in cardiac injury was not addressed. From a report based on the time of measurement and a specific assay, the AUC of troponin I for myocardial infarction diagnosis ranges from 0.82 to 0.96 [17,18]. A multicenter study revealed that this parameter showed 84% sensitivity and 94% specificity for myocardial infarction diagnosis at presentation, which was higher than the diagnostic performance determined in our study [18]. Different pathophysiology and disease definition may have contributed to the different diagnostic performance. The main pathophysiology of myocardial infarction is atherosclerosis, endothelial injury, or plaque rupture. Conversely, cardiac injury induced by CO poisoning arises from the ability of CO to bind to hemoglobin molecules with a high affinity, displace oxygen, and generate carboxyhemoglobin, which cannot effectively deliver oxygen to tissues [13]. Cardiac injury with CO poisoning has various pathogeneses, such as mitochondrial dysfunction, direct injury to myoglobin, and thrombus formation in the microcirculation. Besides hypoxic damage, in combination with cellular or subcellular cardiospecific mechanisms, CO induces myocardial injury by binding to the heme group of myoglobin, reducing oxygen supply to the mitochondria and impairing phosphorylation [19,20]. Strong inhibition of adenosine triphosphate generation forces myocardiocytes to switch to anaerobic metabolism, resulting in hypoxia, lactic acidosis, and apoptosis. All coronary angiograms showed normal coronary arteries in the six patients with increased levels of cardiac biomarkers [6]. Thus, coronary artery stenosis does not represent the main mechanism for cardiac injury development in CO poisoning.
There are several limitations to this study. First, it was a singlecenter, retrospective study with a small sample size. Twenty percent of patients were excluded because of the absence of echocardiography data, which may have affected the study results. Second, visual estimation of ejection fraction can be inaccurate, although several studies demonstrated that it is closely correlated with formal quantitative methods [21,22]. When patients have a normal ejection fraction despite an increased cardiac biomarker level, this may be due to other types of cardiac injury, such as ejection fraction preserved heart failure or right heart dysfunction. Visual estimation may not be suitable to evaluate these conditions. Third, because there was no assessment of previous cardiologic evaluation, it could not be determined whether the cardiac injury was acute or chronic. Additionally, we only have data for coronary artery history; thus, analysis of other factors, such as heart failure, cardiomyopathy, and left ventricular systolic dysfunction, was not conducted. Fourth, owing to the retrospective nature of the study, cases without echocardiography were assumed to be mild cardiac injury. Therefore, our study cohort may include patients with severe cardiac injury, and the results of cardiac biomarker tests and echocardiography may have been overestimated. Fifth, patients who undergo blood tests a long time after the last exposure to CO can have relatively high results compared with those found in actual cardiac injury in which the myocardial biomarker level increases. Because the time between the blood sample time and echocardiography was short, it is possible to examine the relationship between the two tests. Sixth, we also included patients who were involved in fire accidents. Other toxic gases, such as cyanide and hydrogen sulfide, can also induce organ injury in fire accidents in addition to CO poisoning. It appears difficult to distinguish cardiac injury due to CO and that due to other toxic gases in our study. Finally, the prognostic value of cardiac injury was not addressed in this study.
In conclusion, of the cardiac biomarkers, troponin I showed the best diagnostic performance for predicting cardiac injury in CO poisoning, with 67% sensitivity and 91% specificity at a cut-off value of 0.455 ng/mL. Increases above 0.455 ng/mL were associated with cardiac injury and may require further cardiologic evaluation. However, further studies on cardiac biomarkers and other diagnostic tools in CO poisoning are needed given the low sensitivity of troponin I.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Prognosis in patients with cardiac injury related to carbon monoxide poisoning is poor. Therefore, early detection of cardiac injury in patients with carbon monoxide poisoning is very important.
What is new in the current study
Troponin I showed the best diagnostic performance in predicting cardiac injury in patients with carbon monoxide poisoning. A cut-off value of 0.455 ng/mL seemed optimal for predicting cardiac injury. However, further studies on cardiac biomarkers and other diagnostic tools in carbon monoxide poisoning are needed given the low sensitivity of troponin I.