Current updates in acute traumatic aortic injury: radiologic diagnosis and management
Article information
Abstract
Acute traumatic aortic injuries, which have substantial lethal outcomes at the time of admission, are fatal in 80% to 90% of cases. These injuries are relatively rare and have nonspecific clinical presentations. Radiologists and emergency physicians need to identify the radiological signs of acute traumatic aortic injury and differentiate them from common imaging errors to ensure accurate diagnosis and determine appropriate management protocols. In combination with image-guided interventions, advances in cross-sectional imaging have enabled nonsurgical management of acute traumatic aortic injuries. Timely and precise diagnoses of these injuries following prompt treatment are essential as up to 90% of patients presenting at the hospital can undergo early repair.
INTRODUCTION
Acute traumatic aortic injuries (ATAIs), which have substantial lethal outcomes at the time of admission, are fatal in 80% to 90% of cases [1-3]. These injuries are relatively rare and have nonspecific clinical presentations. Male patients, and particularly young male patients between the ages of 30 and 40, are more commonly affected by ATAI [4].
The most common location for an imaged ATAI is the aortic isthmus (approximately 90%), just distal to the origin of the left subclavian artery, because of the relative immobility of the aorta at the site of the ligamentum arteriosum [5,6]. The other sites of ATAI in the thorax are, in descending order of frequency, the ascending aorta/aortic root (5%–8%), aortic arch (2%), and distal descending aorta (1%–12%) [5,7,8]. Injuries that cause aortic tears at more than one location are reported in 6% to 18% of cases [7].
Blunt trauma due to a high-velocity impact, such as a motor vehicle collision, is the most common mode of injury, accounting for 70% of cases, followed by a fall from height [9]. Various mechanisms have been proposed for the processes that lead to ATAI. These include shearing forces, rapid deceleration, hydrostatic forces, and the osseous pinch [5,9]. Rapid deceleration in the anteroposterior and lateral directions results in torsion and shearing forces against the aorta at relatively immobile levels. Another mechanism is a sudden increase in intra-aortic pressure, which can exceed 2,000 mmHg, following direct compression [10]. This has been termed the “water-hammer effect,” and results primarily in horizontal tears of the isthmus but may also show retrograde extension with injury at the aortic root [11]. In the osseous pinch mechanism, anterior chest-wall bones (clavicles, ribs, and the manubrium) directly compress the aorta against the spine [12]. Direct injury to the aorta can occur in penetrating trauma as gunshot or knife wounds with severity related to the trajectory of the weapon [13,14]. Knowledge of the mode of injury that may result in an ATAI should warrant radiological imaging.
Cases of ATAI of the abdomen account for 11% to 15% of aortic injuries [14]. The frequency of abdominal aortic injuries is much lower than that of thoracic aortic injuries because the abdominal aorta is relatively well protected [14-16]. The divisions of abdominal aorta most commonly involved with trauma are, in descending order of frequency, infrarenal (67%), suprarenal (33%), and extension from a thoracic aortic injury (25%) [16]. Abdominal aortic injuries are frequently a result of a crushing injury between the lumbar spine and lap belt. Shearing forces do not play a major role in abdominal aortic injuries [7]. Abdominal aortic injury patients commonly have neurological deficits ranging from sensory loss to paraplegia [7].
ETHICS STATEMENT
As a teaching institute, All India Institute of Medical Sciences obtain consent from all patients prior to all procedures and other management for using their data for educational and teaching purpose. Also, we have neither used any clinical image nor revealed any other patient information as all radiology images are anonymized.
CLINICAL PRESENTATION
Clinical features are often unreliable for diagnosis or exclusion of ATAI. Patients may present with nonspecific and variable clinical signs and symptoms, such as chest pain, back pain, breathing difficulty, external chest-wall injuries, and increased chest-drain output, depending on the mechanism and magnitude of the injury [17]. In fact, the signs of aortic damage may not be recognized clinically until sudden hemodynamic instability. Hypotension, upper-limb hypertension, and lower-limb hypotension with reduced femoral pulses (pseudocoarctation syndrome), and differences in blood pressure between the right and left brachial arteries are warning signs suggestive of aortic injury. However, these signs are absent in up to one-third of patients [5,13,18]. An evaluation of concomitant visceral injuries that may be immediately life-threatening is essential and should take priority over management of aortic injury. Attention to fractures of the sternum and first and second ribs, clavicle, and/or scapular fractures, pneumothoraces, hemothoraces, flail chest, pulmonary contusions, diaphragm injury, tracheobronchial disruption, and esophageal injuries in high-impact trauma is essential for the effective management of high-risk ATAI. Other common concomitant injuries are severe head injury, lung and cardiac injury, diaphragmatic rupture, intra-abdominal bleed, pelvis trauma, and long bone fractures [9].
DIAGNOSTIC IMAGING
Chest X-rays
A supine chest X-ray (CXR) offers an adjunct to primary assessment and is the first line of imaging in acute trauma [19]. It can diagnose immediate life-threatening conditions such as tension pneumothorax or large hemothorax, and evaluate the position of tubes and lines [13,14,20]. Although CXR has high sensitivity of 90% in detecting mediastinal hemorrhage, the specificity is low, as there are other causes of mediastinal hematoma [21]. A CXR can rule out ATAI in most cases, however a small percentage (0.5%–7%) of patients with normal CXRs may actually have ATAI [22]. Patients suspected to have suffered an ATAI should undergo further imaging [21].
Radiographic signs in acute traumatic injury of thoracic aorta are as follows (Fig. 1) [13,14,20,21]. A widened mediastinum of more than 8 cm and/or 25% of the thoracic width (at the level of the aortic knob) is the most frequent observation. It is highly sensitive (81%–100%) and can detect mediastinal hematoma, but the specificity is low (34%–60%) as venous bleeds and sternal and vertebral fractures can also lead to mediastinal bleeds [22]. This technique is also associated with false positivity because the supine position and anteroposterior view lead to projectional variability of the mediastinal silhouette.

Chest X-ray depicting mediastinal widening (double arrow) at the level of the aortic knob, along with obscuration of aortic contours (arrowheads) in a posttraumatic patient. Note the right paratracheal stripe widening (asterisk) and the depression of left mainstem bronchus (black arrow). The findings are highly indicative of aortic injury.
Loss of aortic knob or aortopulmonary window contour is the most consistent and reliable sign of ATAI of the thoracic aorta. Cases without mediastinal widening, but with loss of the sharp interface of the lung and the transverse or descending thoracic aorta, should be considered suspicious, as aortic contours should have a well-defined interface with the adjacent lung and aortopulmonary window. When applied in conjunction with mediastinal widening, this radiographic sign provides superior diagnostic accuracy.
Depressed left mainstem bronchus (more than 40 degrees from the horizontal), deviated trachea, or support devices, including endotracheal and enteric tubes toward the right (to the right of the spinous process of the third or fourth dorsal vertebra) are also indicative of ATAI and manifest due to the mass effect of mediastinal hematoma [22].
The left apical pleural cap is an occasionally seen in ATAI and occurs due to bleeds coursing along the left subclavian artery reflection [5,22]. The left apical cap is reportedly not specific for aortic injuries, and other causes, such as pleural thickening, hematomas from central venous pressure-line placement, rib fractures, clavicular fractures, or subclavian arterial trauma, must be considered. Thickening by more than 5 mm of the right paratracheal stripe and the left hemothorax may be present in ATAI.
A combination of these positive signs add to the diagnostic value of CXR for ATAI. In a patient with a high-energy impact, mediastinal widening with loss of aortic contours on CXR is highly indicative of ATAI and warrants further investigation with multidetector computed tomography (MDCT) and CT angiography (CTA).
Focused assessment with sonography for trauma
Focused assessment with sonography for trauma (FAST) can detect hemothorax and pericardial effusion, which may occur in the event of a free aortic rupture. However, FAST has low sensitivity and specificity in detecting ATAI [23].
Multidetector computed tomography
With the advent of modern technology, MDCT has superseded digital subtraction angiography (DSA) for the detection of ATAI [17]. MDCT with CTA is now the tool of choice for detecting aortic injury, offering 98% sensitivity and 100% specificity in the evaluation of suspected ATAI [9]. Contrast-enhanced MDCT is favored for assessments of polytrauma patients and is considered an appropriate imaging modality that can reliably exclude ATAI, with a negative predictive value approaching 100% in some studies [12]. Few studies have suggested that single-phase contrast-enhanced CT provides comparable accuracy [24]. Electrocardiogram gating is not mandatory in trauma settings as setup times can be lengthy and require patients to hold their breath for extended periods [9]. The drawbacks associated with motion-related artifacts at the aortic root and heart and breathing artifacts have largely been overcome by ultrafast acquisition in newer dual-energy CT machines [9]. The acquired CT scans are then analyzed on three dimensional workstations to evaluate aortic injuries using multi-planar reformations, which provide information on the extent and grade of injury and depict the relationship and distance of the injury to the left subclavian artery [24]. An ATAI is best visualized in the sagittal-oblique plane of the thoracic aorta, simulating a projection obtained from conventional angiography [25].
CT Signs of ATAI
Most ATAIs (approximately 90%) occur along the antero-medial aspect of the aortic isthmus [7]. The CT signs of ATAI can be either direct or indirect, based on the presence or absence of signs of alteration of the aortic wall itself, as summarized in Table 1 [8]. The diagnostic accuracy of CT has been found to be 100% in the presence of direct signs [12]. Patients with direct signs of ATAI on MDCT require no further imaging to avoid delays in timely management.
Direct signs are defined on the basis of degree of injury to aortic wall with intimal, medial, and adventitial injuries displaying different radiological manifestations [4,13,14]. These include the presence of an intimal flap, intraluminal and mural thrombus, focal-wall outpouching, aortic dissection, abnormal aortic contours, sudden changes in aortic caliber (aortic “pseudocoarctation”), and periaortic contrast extravasation [4,13,14].
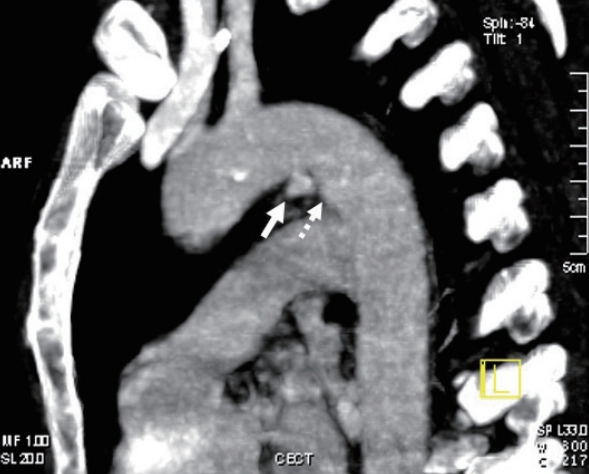
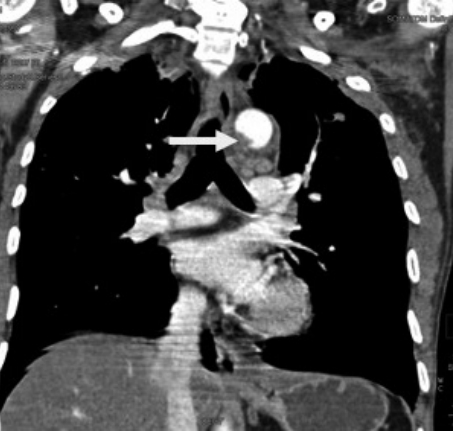
An intimal flap is seen as a linear filling defect within the aortic lumen as a result of an intimal tear (Fig. 2) [17]. An intraluminal thrombus appears as a globular, intraluminal filling defect representing a thrombus secondary to intimal damage (Fig. 3) [14]. An intramural hematoma is visualized as a hyperdense crescent in the aortic wall (Fig. 4) [9].

Intimal flaps in aorta. An intimal flap can be seen as a sharp, linear filling defect (arrow) in two different patients in (A) thoracic aorta and (B) abdominal infrarenal aorta.
An intraluminal thrombus in the aorta. An intraluminal thrombus can be seen as a globular, filling defect within the aortic lumen (arrow).
Intramural hematoma in the aortic wall. It presents as a crescent-shaped density in the aortic wall (arrow) in (A) axial and (B) sagittal oblique images.
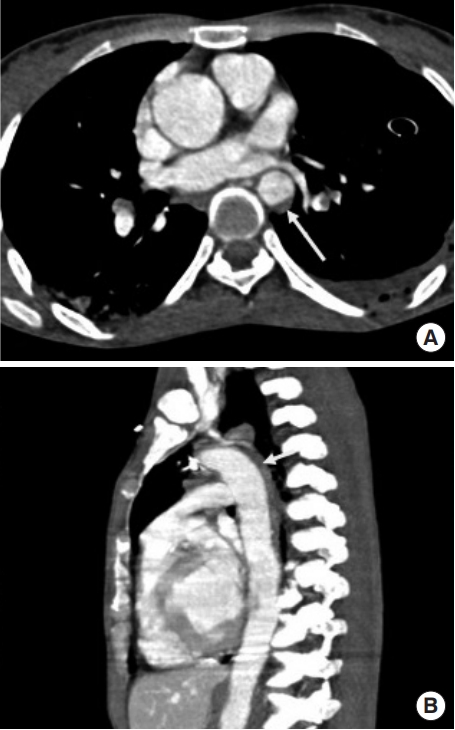
Focal-wall outpouching or an abnormal aortic contour (pseudoaneurysm), as depicted in Fig. 5, reflects focal damage to both the tunica intima and media, with adventitia remaining as the outermost intact layer retaining blood [17]. Focal-wall outpouching is the result of the highly compliant nature of the adventitia, which is at risk of rupture if not treated. Harris et al. [26] developed a risk score for assessing aortic stability and prognostications regarding rupture; the parameters include admission lactate >4 millimoles, pseudoaneurysm/normal aortic diameter ratio >1.4 (Fig. 6), and mediastinal hematoma thickness along the descending thoracic aorta >10 mm. They concluded that there is an increased risk of aortic rupture in the presence of any two of these factors [26]. Untreated or undiagnosed injuries can develop into chronic pseudoaneurysms in 1% to 2% of patients [6], who may be thrombosed with dense peripheral calcification [25].
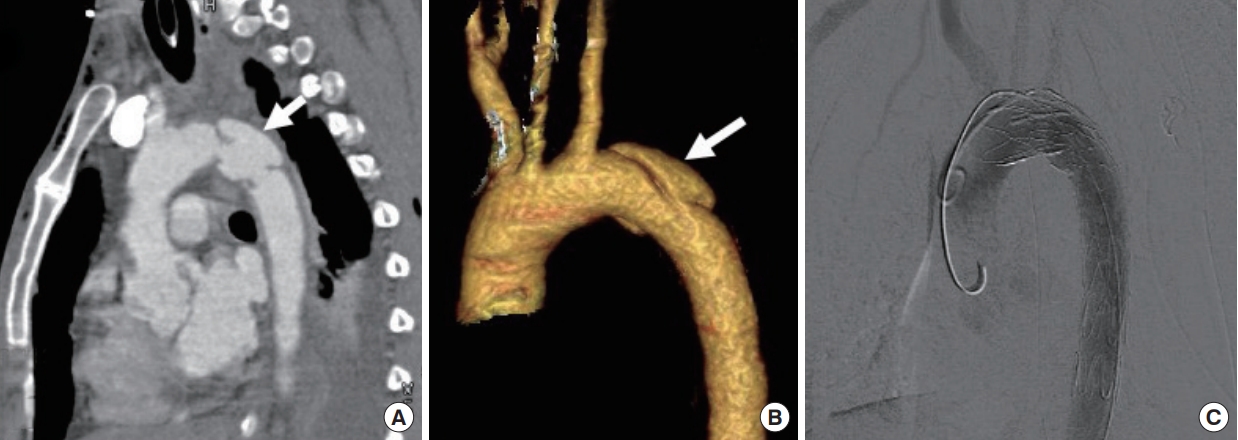
A pseudoaneurysm. An abnormal aortic contour with focal bulge (arrow) just distal to subclavian artery in (A) maximum intensity projection oblique sagittal images and (B) volume rendering technique images. (C) This case was treated with endovascular stent graft placement.
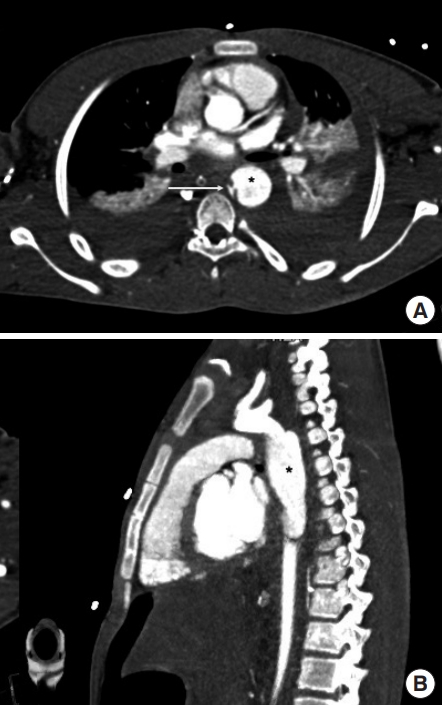
Pseudoaneurysm with pseudocoarctation. A focal-contrast-filled outpouching (asterisk) leading to a significantly compressed aortic lumen (arrow) in (A) axial multidetector computed tomography images and (B) oblique sagittal maximum intensity projection images. A pseudoaneurysm/normal aortic diameter ratio >1.4 is a predictor for rupture.
Periaortic contrast extravasation is the most apparent direct sign and represents a tear of the full thickness of the aorta (Fig. 7). It is essential to understand that the absence of active extravasation does not exclude an aortic transection, as intraluminal contrast within a completely transected aorta remains within the confines of the periaortic connective tissue.

Periaortic contrast extravasation. (A) Axial and (B) coronal reformatted computed tomography images in the arterial phase show an ill-defined focus of extravasated contrast matching the blood pool (arrow) in a retroaortic location with significant retroperitoneal hemorrhaging. In (C) the corresponding axial and (D) coronal reformatted portovenous phase images, the extravasated contrast (arrow) is shown expanded and spreading around the aorta.
Indirect signs include periaortic hematoma (mediastinal hematoma in the thorax and retroperitoneal hematoma in the abdomen), as depicted in Fig. 8, and are usually a result of the avulsion of perivascular veins from the arterial vasa vasorum rather than extravasation of intraluminal blood from the vessel itself [4,13,14,25]. In an ATAI, the hematoma is in direct contact with the aorta, with an indistinct intervening fat plane between hematoma and aorta. This finding helps exclude mediastinal hematoma due to venous mediastinal bleeding, sternal/vertebral body fractures, or intercostal arteries hematoma [4,14,25]. In the presence of such periaortic hematomas, physicians should look for signs of direct aortic injury. However, an ATAI may be present despite nonvisualization of periaortic mediastinal hematoma when the injury is confined to the intima. Periaortic hematoma in the absence of direct signs of aortic injury could be due to an occult intimal injury. Intravascular ultrasound or transesophageal echocardiography can supply definitive details in such cases [9].

Periaortic hematoma surrounding the aorta. Periaortic hematoma (arrowheads) can be seen closely surrounding the aorta with no intervening fat in (A) and (B). A closer look leads to a tiny focal pseudoaneurysm (arrows) as seen in (A) axial, (B) oblique sagittal, and (C) volume rendering technique images.
Pitfalls of CT angiography
Imaging errors can be technical or anatomical. Technical pitfalls include pulsation, breathing, and motion artifacts [8]. Pulsation artifacts are usually seen at the aortic root and in the ascending aorta and mimic intimal flaps (Fig. 9). Traumatic intimal flaps have sharp margins while those arising due to artifacts have fuzzy margins. Simultaneous artifacts in the main pulmonary artery can also help distinguish a true injury from an artifact. An electrocardiogram-gated CT scan can be performed in cases in which dilemmas cannot be solved [13]. An artifact due to movement or breathing mimics an intimal flap and appears to be projecting over the lumen and adjoining mediastinal fat or lung parenchyma.
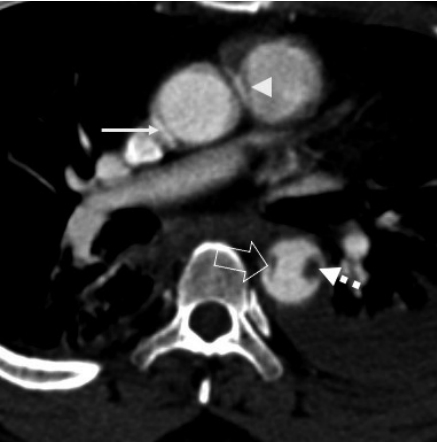
A pulsation artifact at the aortic root (arrow) mimicking an intimal flap. The fuzzy margins and simultaneous artifact in the main pulmonary artery (white arrowhead) helps distinguish the true injury from artifacts. Also seen in the same section is a traumatic intimal flap (blank arrow) with sharp margins in the descending aorta. An intraluminal thrombus (dashed arrow) is adjacent to it.
Several anatomical variants can cause confusion in assessments of ATAI. The most common anatomical variant is ductus diverticulum. This is an embryonic ductus arteriosus remnant. A ductus “bump” has been reported in approximately 9% of the general population [4]. Differentiation of ductus diverticulum from a traumatic focal contour bulge is critical as both are seen at the site of the aortic isthmus (Fig. 10). Ductus diverticulum is usually present in the inferior aortic arch at the level of the aortic isthmus and has a smooth focal bulge, a broad neck, and gentle obtuse angles with the aortic wall [14]. This is in contrast to traumatic focal contour bulges, which usually form sharp margins with the aorta and are often associated with signs of regional trauma, such as an intimal flap or periaortic hematoma. The presence of calcification favors ductus diverticulum over an ATAI [14]. Other anatomical structures that can introduce diagnostic uncertainty include the aortic spindle and the ostium of branch arteries. The aortic spindle has a fusiform dilated appearance and is distal to aortic isthmus. As a nonpathological dilatation of bronchial, intercostal, lumbar, and sacral arteries, ostia may mimic a tiny pseudoaneurysm; however, the appearance of an artery at the distal end of the conical ostium can help reach a diagnosis. Simultaneous opacification of the aorta and adjacent veins (hemiazygos, intercostal, or bronchial veins) often results in a false appearance of an intimal flap [4]. Enhancement of a small and collapsed adjacent lung may also simulate an intimal flap; however, tracing pulmonary vessels and bronchi into the collapsed lung can confirm the diagnosis [4]. While residual thymic tissue can mimic a hematoma, the characteristic triangular shape of thymic tissue and the absence of accompanying fat stranding can help reach a conclusion. Pericardial recesses can also be mistaken for mediastinal or intramural hematoma. Fluid attenuation and the preserved fat plane between the recess and the aorta can help distinguish this normal structure from a mediastinal hematoma. Knowledge of the location of anatomical structures and scrolling through sequential images should eliminate diagnostic confusion.
Digital subtraction angiography
Historically, DSA was considered the gold standard to diagnose ATAI, with a sensitivity of nearly 100%, a specificity of greater than 98%, and accuracy of more than 99%. However, with the advent of MDCT and its high ATAI sensitivity and specificity, DSA is now reserved for endovascular intervention or patients with lateralizing signs of ATAI but lacking definitive signs on CT. The signs of ATAI on DSA are vessel-wall irregularity, including breaches in continuity, contrast-material extravasation in the event of complete rupture, pseudoaneurysm, and pseudocoarctation [4]. Transesophageal echocardiography can be used bedside if the patient is unstable and other examinations are impossible.
Magnetic resonance imaging (MRI) has a limited role in the detection of ATAI due to long acquisition time, the need for patient immobility, and difficulty introducing certain support systems into an MRI room. However, because of the advantage of MRI characteristics in detecting aortic trauma, it can be useful in monitoring intimal injuries, follow-ups after endovascular stent placement, delayed elective surgical repair, and, in young patients, reducing radiation exposure [9].
Intravascular ultrasound is a helpful adjunct in scenarios in which CTA findings are equivocal because it provides cross-sectional images of the vessel wall and adjoining tissues at high resolution [9]. Angiography may not reveal any abnormality in approximately 50% of patients, and intravascular ultrasound may help reach a proper diagnosis [7].
CLASSIFICATION OF ACUTE TRAUMATIC AORTIC INJURIES
The importance of classifying injuries lies in identifying features that point to a poor prognosis, underlining the importance of correct use of MDCT signs. Various classification systems have been used in recent years to define the severity of ATAI, the most widely used of which is Society of Vascular Surgery (SVS) classification [27].
Direct signs of ATAI detected on MDCT can be classified according to SVS as grade 1 (intimal flap), grade 2 (intramural hematoma), grade 3 (pseudoaneurysm), and grade 4 (rupture), based on anatomical layers of the aortic wall [28].
The Harborview classification simplifies the SVS grading criteria of ATAI into minimal, moderate, and severe categories, on the basis of differences in treatment among these three categories (Table 2) [29].
Minimal aortic injury (MAI) includes SVS grade 1 and 2 injuries, with no external contour abnormality and an intimal tear, intraluminal or intramural thrombus, or both, <1 cm in size, with no to minimal mediastinal hematoma [29,30]. These injuries are associated with a good prognosis and should be considered for nonoperative management with close follow-up as they are known to heal spontaneously [23]. Approximately one-third of MDCT-diagnosed blunt ATAIs are of a minimal grade [9,18].
Moderate aortic injuries include larger intimal tears, larger intraluminal and intramural thrombus (>10 mm) or a pseudoaneurysm [29]. An intimal flap or thrombus >1 cm in size is important in differentiating minimal from moderate aortic injuries as half of intimal flaps >1 cm are known to progress to a higher injury grade within 8 weeks, at which point they will require repair [9,17]. Endovascular repair or surgery is standard for definitive treatment in this category of patients and its timing depends on the hemodynamic stability of the patient [31]. Severe aortic injuries include active-contrast extravasation or a left subclavian artery hematoma >15 mm and should be repaired immediately [29].
MANAGEMENT
Conservative
Treatment of all patients with ATAI begins with anti-impulse therapy (beta-blockers), antiplatelets, and anticoagulant agents [18]. The goal is to maintain a mean arterial pressure ≤80 mmHg, systolic blood pressure between 100 and 120 mmHg, and a heart rate between 60 and 80 beats/min. To prevent the injury from progressing further, aortic-wall stress should be reduced, unless contraindicated by concurrent injuries such as traumatic brain or spinal cord injuries that may require elevated blood pressures to maintain adequate tissue perfusion. Such measures drastically decrease the chance of rupture to <2% [23]. Calcium channel blockers or arterial vasodilators are additionally used when further supplementation is required [9]. Medical management is either temporary (for moderate and severe aortic injuries) or definitive (for MAIs).
A follow-up CT scan within 48 to 72 hours is recommended for patients with MAI to establish stability and/or resolve the abnormality. Endovascular repair can be used to treat any progression during follow-up imaging. Heneghan et al. [29] suggested that follow-up imaging is not needed for such patients, whereas Gunn et al. [32] advised follow-up imaging be performed at 1, 3, 6, and 12 months postinjury, with subsequent yearly cardiovascular MRI scans. Surveillance for such patients typically ends when the aorta returns to a normal appearance [9]. Most studies have shown that progression of disease is rare, and has been found to occur only in the first month after injury [9].
Endovascular versus open repair
Endovascular repair (Fig. 11) has gained popularity over open surgical repair, and is now the first line of treatment if it is technically feasible [24,33]. The morbidity associated with a thoracotomy, aortic cross-clamping, and cardiopulmonary bypasses can be avoided with endovascular repair [8,34]. Endovascular repair prevents further progression of ATAI by excluding the aorta from systemic blood pressure. Citing lower risks of morbidity, mortality (9% vs. 19%), and spinal cord ischemia (3% vs. 9%), SVS clinical practice guidelines recommend endovascular repair over open repair for all age groups with suitable anatomy [35]. Patients undergoing endovascular repair have been found to experience reduced complications and earlier hospital discharge compared with those treated with open repair [9].
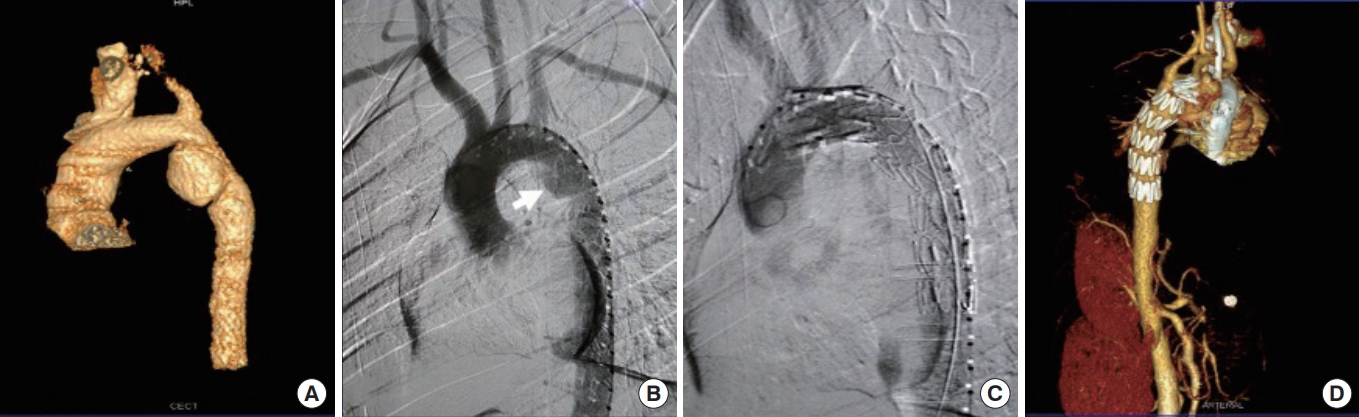
A pseudoaneurysm in the descending thoracic aorta and its images after stent placement. (A) An multidetector computed tomography volume rendering technique image revealing a pseudoaneurysm in descending thoracic aorta distal to the left subclavian artery with (B) a corresponding aortogram revealing the same (arrow). The patient underwent successful covered stent graft placement, as seen in (C) aortogram, which depicts normal aortic contour poststent placement. (D) A corresponding multidetector computed tomography volume rendering technique images reveal normal aortic contour poststenting.
Open repair continues to be required in cases with anatomic variations that are incompatible with an endovascular approach. Such variants involve arch type, tortuosity, the diameter of proximal landing zone, and iliac vessel size [23]. Open repairs last longer than endovascular repairs and require less re-intervention; however, their early morbidity and mortality is considerably higher [21]. Cases of ATAI in abdomen are localized into one of three anatomical zones that determine whether endovascular repair is possible: zone I (diaphragmatic hiatus to superior mesenteric artery [SMA]), zone II (SMA through the renal arteries) and zone III (renal arteries to the aortic bifurcation) [36]. Zone I injuries require extensive open exposure, but could be amenable to endovascular repair [37,38]. Zone II injuries are not amenable to endovascular stent placement, as fenestrated grafts customized to accommodate the SMA and renal arteries are not well suited for use in acute settings. Zone III injuries are amenable to open or endovascular repair [39]. Bowel injury must be ruled out to avoid infection of open grafts because of the intraperitoneal free succus or stool. Such scenarios can be managed with endovascular repair.
Timing of repairs
Severe aortic injuries require immediate repair, while repair of moderate aortic injuries is undertaken after management of accompanying injuries and patient stabilization before aortic intervention, which may account for improved outcomes [23]. A study comparing patients who underwent early (<24 hours) repair compared to delayed (>24 hours) repair concluded that mortality was remarkably reduced in the patients with delayed repair in comparison to the early repair group (5.8% vs. 16.5%) for this reason [27].
MANAGEMENT ALGORITHM
Technical aspects
The following must be documented on MDCT for planning of endovascular stent grafting.
Type of injury
The length of the vascular injury and diameter of the aorta cranial and caudal to the site of injury on sagittal oblique multiplanar reformations or curved reformatted images should be documented. Overestimation of stent size by at least 15% is essential to prevent endovascular stent leakage. This is a potential problem, particularly in hypovolemic patients, as underestimates of endoprosthesis caliber may occur because of vasoconstriction in hemorrhagic shock, leading to small aortic caliber [24,40]. Details regarding the proximal and distal landing zone with specific information regarding landing zone length, which should be at least 2 cm, are crucial landing zone calcification if present should also be documented.
Distance from left subclavian artery to the injury
This needs to be measured as a left subclavian artery origin should be covered with a subsequent left carotid-subclavian artery bypass in cases with a small proximal landing zone. An angiography of the aortic arch branches therefore becomes indispensable in assessing circulation in the circle of Willis, particularly before the intentional occlusion of the left subclavian artery, without prior stenting [18]. Prophylactic embolization of the proximal left subclavian artery can be delayed in zone 2 thoracic endovascular aortic repair (TEVAR) due to ATAI, unlike TEVAR performed for an aneurysm. This is because ATAI is an emergency requiring prompt TEVAR, whereas subclavian artery embolization is a preventative procedure to prevent type II endoleakage [38]. These procedural details in traumatic zone II TEVAR are also confirmed in Figs. 5B, C and 11C, D.
Anatomic variants such as arch anatomy (type I, II, III), direct vertebral artery origin and aberrant subclavian artery (e.g., occlusion of aberrant right subclavian artery) have to be determined prior to endoprosthesis placement in the descending thoracic aorta [14]. Other important elements include vertebral artery dominance [14] and tortuosity of the aorta. Existence of atherosclerotic disease or stenosis and prior operative changes, such as a coronary artery bypass graft, should also be noted.
External and common iliac artery diameters and tortuosity
Reduced diameter of access vessels may dramatically alter the appropriate approach to endovascular repair. An iliac artery diameter <7 mm has been considered a limiting factor [23]. A tortuous course of the iliac-femoral axis has not been considered a contraindication of aortic stenting.
Treatment and outcomes of traumatic aortic injuries in younger populations can be problematic because of the smaller aortic caliber, which can complicate graft sizing [41]. Aortic arch geometry is more pointed in younger patients and may have poor compliance of the endograft to the aortic arch [9].
Monitoring
Minimal aortic injuries should be closely monitored by CTA after 48 to 72 hours and later on as already described. Angiography with MRI has been recommended for further follow-up.
Patients who undergo endovascular repair should also undergo graft surveillance. This is particularly important in younger patients to ensure endograft stability and integrity because the aorta remodels and lengthens with age. Aortic dilatation is more pronounced at the site of implantation and in patients receiving a stent for traumatic injury repair compared with aneurysm repair. Radiological surveillance has been recommended to be performed at 1 and 6 months, annually for the first 5 years, and then every 2 to 3 years on case-by-case basis [8]. A CTA should be used for initial examinations, and MRI can be utilized for follow-up examinations [8].
CONCLUSION
ATAI is a potentially lethal entity, the outcome of which is highly dependent on early clinical suspicion, radiologic diagnosis of aortic injury, and appropriate management, including endovascular repairs. Radiologists and emergency physicians require access to direct MDCT findings based on the grading and management of aortic injuries chosen and evaluate concomitant visceral injuries that may be more life-threatening than the aortic injury itself.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Acute traumatic aortic injuries are associated with substantial lethal outcome at the time of admission fatal in 80% to 90% of cases. These injuries are relatively rare and have nonspecific clinical presentation. Early identification of the radiological signs of acute traumatic aortic injuries and differentiation from common imaging pitfalls are essential to facilitate the diagnosis of these injuries and help decide the management protocol.
What is new in the current study
Our article provides a comprehensive and updated review on the diagnosis and management of acute traumatic aortic injury, which will be useful for both emergency physicians and radiologists.