Comparison of intracranial pressure changes in out-of-hospital cardiac arrest patients with and without malignant blood-brain barrier disruption
Article information
Abstract
Objective
In the present study, intracranial pressure (ICP) changes were investigated in out-ofhospital cardiac arrest (OHCA) patients with and without malignant blood-brain barrier (BBB) disruption who underwent target temperature management.
Methods
This prospective, single-center, observational study was conducted from June 2019 to December 2021. ICP and albumin quotient values were measured on days 1, 2, 3, and 4 of hospitalization. Malignant BBB disruption was defined as the sum of scores for the degree of BBB disruption ≥9 on days 1 to 4.
Results
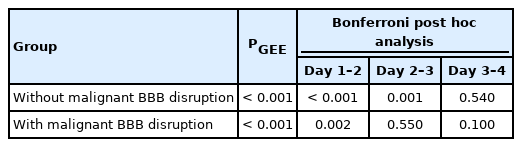
ICP in OHCA patients without malignant BBB disruption on days 1, 2, 3, and 4 of hospitalization was 9.58±0.53, 12.32±0.65, 14.39±0.76, and 13.88±0.87 mmHg, respectively, and in OHCA patients with malignant BBB disruption 13.65±0.74, 15.72±0.67, 16.10±0.92, and 15.22±0.87 mmHg, respectively (P<0.001, P<0.001, P=0.150, and P=0.280, respectively). The P-values of changes in ICP between days 1 and 2, days 2 and 3, and days 3 and 4 of hospitalization in OHCA patients without malignant BBB disruption were P<0.001, P=0.001, and P=0.540, respectively, and in OHCA patients with malignant BBB disruption were P=0.002, P=0.550, and P=0.100, respectively.
Conclusion
Among OHCA patients treated with target temperature management, ICP was higher on days 1 and 2 of hospitalization and an increase in ICP occurred earlier with malignant BBB disruption than without malignant BBB disruption.
INTRODUCTION
The blood-brain barrier (BBB) regulates the entry of substances in plasma into the brain and is composed of microvascular endothelial cells, a capillary basement membrane, pericytes, and astrocyte endfeet. Endothelial cells form junctional complexes with tight junctions and adherens junctions [1-3]. BBB disruption can occur in Alzheimer’s disease, infection, traumatic brain injury, stroke, cancer, and epilepsy [4-7]. Neurotoxic materials in the plasma enter the central nervous system through the disrupted BBB and cause neuronal injuries [8,9].
Hypoxic ischemic brain injury after cardiac arrest (CA) increases the BBB permeability due to microvascular damage induced by oxidative stress, resulting in BBB disruption [10]. In cytotoxic edema, the total brain tissue mass remains unchanged; however, in vasogenic edema resulting from BBB disruption, water is added from the vascular space, which leads to tissue swelling, tissue movement, and, eventually, elevated intracranial pressure (ICP) [11,12]. Cerebral edema and elevated ICP lead to reduced cerebral blood flow, resulting in a vicious cycle of cerebral injury due to decreased cerebral blood flow [13-16].
In previous studies, moderate or severe BBB disruption was strongly associated with poor neurological outcomes, and a BBB disruption score ≥9 had poor neurological outcomes in survivors of out-of-hospital CA (OHCA) who had undergone target temperature management (TTM) [17,18]. Sekhon et al. [19] reported a relatively low burden of ICP, however, most CA patients demonstrated an increased compensatory reserve index, indicating a state of limited intracranial compensatory reserve and compliance in hypoxic ischemic brain injury after CA.
To the best of our knowledge, ICP with BBB disruption scores ≥9 (defined as malignant BBB disruption) has not yet been investigated. Therefore, ICP changes in OHCA patients with and without malignant BBB disruption who underwent TTM were investigated in the present study. The results can be used as baseline ICP data in future studies aimed at diminishing malignant BBB disruption.
METHODS
Ethics statement
This study was approved by the Institutional Review Board of Chungnam National University Hospital (No. CNUH IRB 2019-07-033-09). All procedures and protocols were conducted in accordance with the Declaration of Helsinki and the International Conference of Harmonisation Good Clinical Practice and reported as CONSORT (Consolidated Standards of Reporting Trials) criteria. Approval and written informed consent from the patients’ next of kin were obtained prior to study enrollment. The research method used in previous studies was followed in the present study [20,21].
Study design and patients
This study was a single-center, prospective, observational cohort study of OHCA patients treated with TTM between June 2019 and December 2021. For the primary endpoint, ICP changes in OHCA patients with and without malignant BBB disruption who underwent TTM were investigated. The patients’ caregivers were called 6 months after the return of spontaneous circulation (ROSC) to obtain patient neurological outcome data. A cerebral performance category (CPC) of 1 to 2 demonstrated good neurologic outcomes and a CPC of 3 to 5 was associated with poor neurologic outcomes. Resuscitated OHCA patients who were treated with TTM and whose Glasgow Coma Scale (GCS) score was ≤8 after ROSC were included in this study. The exclusion criteria were as follows: (1) <18 years of age, (2) CA due to trauma or TTM interruption due to hemodynamic instability, (3) ineligibility for TTM (i.e., brain hemorrhage, active bleeding, known terminal illness, or poor neurological status before CA), (4) ineligibility for lumbar puncture (i.e., severe cerebral edema on brain computed tomography, loss of the basal cisterns, intracranial mass, antiplatelet therapy, anticoagulation therapy, or coagulopathy; platelet count <40×103/mL or international normalized ratio >1.5) [22], (5) receiving extracorporeal membrane oxygenation, (6) refusal of lumbar puncture by the kin, and (7) cases in which the next of kin refused further treatment.
TTM protocol
TTM was performed using cooling devices (Arctic Sun Energy Transfer Pads; Medivance Inc., Louisville, CO, USA). The target temperature of 33°C was maintained for 24 hours with subsequent rewarming to 37°C at a rate of 0.25°C/hr, and the temperature was monitored using esophageal and bladder temperature probes. Midazolam (0.05 mg/kg intravenous bolus, followed by a titrated intravenous continuous infusion at a dose between 0.05 and 0.2 mg/kg/hr) and cisatracurium (0.15 mg/kg intravenous bolus, followed by an infusion up to 0.3 mg/kg/hr) were used for shivering control and sedation, and anesthesia depth was monitored using the Anesthetic Depth Monitor for Sedation (Unimedics Co., Seoul, Korea). Electroencephalography was performed for persistent deterioration of the patient’s level of consciousness, involuntary movements, or seizures. Patients with electrographic seizures or clinically diagnosed seizures were treated with antiepileptic drugs (levetiracetam; loading dose: a 2-g bolus intravenously, maintenance dose: a 1-g bolus intravenously twice daily). Fluids or vasopressors were administered when necessary to maintain the mean arterial pressure between 85 and 100 mmHg [23].
Data collection
The following data were collected: age, sex, CA witness, bystander cardiopulmonary resuscitation (CPR), first monitored cardiac rhythm, etiology of CA, time from ROSC to reaching the target temperature of 33°C (induction time), time from ROSC to measuring ICP via lumbar puncture (ICP time), time from collapse to CPR (no flow time), time from CPR to ROSC (low flow time), sequential organ failure assessment, GCS scores after ROSC, and CPC at 6 months after ROSC.
Measurement of ICP and Qa
The ICP was measured after the patient was placed in the lateral decubitus position and a lumbar catheter inserted using a Hermetic lumbar accessory kit (Integra Neurosciences, Plainsboro, NJ, USA) at the level between the third and fourth lumbar vertebrae of the patient with hip and knee flexed [24]. ICP was continuously measured using a LiquoGuard pump system (Möller-Medical, Fulda, Germany) and taken as the average of the values measured for 1 hour before obtaining cerebrospinal fluid (CSF). Serum blood samples were obtained via venipuncture. Serum and CSF samples were obtained on the first day of hospitalization and on every subsequent day of hospitalization as follows: CSF serum albumin quotient (Qa) values were calculated on days 1 (Qa1), 2 (Qa2), 3 (Qa3), and 4 (Qa4) of hospitalization. BBB disruption was defined as normal (Qa, ≤0.007), mild (Qa, 0.007 to 0.01), moderate (Qa,0.01 to 0.02), or severe (Qa, ≥0.02) [25]. Based on a previous study, malignant BBB disruption was defined when the sum of the scores for the weighted degree of BBB disruption was ≥9 on days 1 to 4 (for example, 02 [normal, day 1]+12 [mild, day 2]+22 [moderate, day 3]+32 [severe, day 4]=14) [18].
Statistical analysis
Continuous variables were described as medians with interquartile ranges or means and standard deviations depending on the normal distribution. Categorical variables were described as frequencies and percentages. The two groups were compared using the chi-square test and Fisher exact test. The correlation between Qa, and ICP was analyzed using Kendall’s tau. The changes in ICP levels over time were analyzed using the generalized estimating equation and Bonferroni post hoc test based on the presence or absence of malignant BBB disruption. All statistical analyses were performed using PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) and MedCalc ver. 15.2.2 (MedCalc Software, Ostend, Belgium). Results were considered statistically significant when the P-value was less than 0.05.
RESULTS
Characteristics of study subjects
Among the 148 OHCA patients in whom ROSC was recorded, 62 patients were enrolled in this study: 26 patients without malignant BBB disruption and 36 with malignant BBB disruption (Fig. 1). In the patients without malignant BBB disruption, 21 subjects (80.8%) had good neurological outcomes, and in the patients with malignant BBB disruption, seven (19.5%) had good neurological outcomes. Furthermore, 23 patients underwent delayed percutaneous coronary intervention for the evaluation of acute myocardial infarction as the cause of OHCA after TTM in the present study. Differences were not observed between the core temperature measured using an esophageal or bladder temperature probe. Complications associated with the lumbar drainage catheter, including bleeding, infection, or brain herniation, did not occur in the enrolled patients. Significant differences were not observed between the patients with and without malignant BBB disruption in terms of mean age, sex, presence of a witness, bystander CPR, cardiac etiology, ICP time, induction time, no flow time, and sequential organ failure assessment scores (Table 1). Among the 62 enrolled patients, 22 (35.5%), 6 (9.7%), 1 (1.6%), 20 (32.3%), and 13 (21.0%) had a CPC of 1, 2, 3, 4, and 5, respectively. A CPC of 5 was observed in 13 patients (21.0%) with conservative management after completion of TTM. Among the 62 enrolled patients, 10 patients died after organ donation and five died of pneumonia.

Flow chart of the study. ROSC, return of spontaneous circulation; GCS, Glasgow Coma Score; ECMO, extracorporeal membrane oxygenation; BBB, blood-brain barrier
Correlation of ICP and Qa in patients with and without malignant BBB disruption
Overall, correlation coefficients between Qa and ICP were 0.62 (95% confidence interval [CI], -0.30 to 0.47), 0.14 (95% CI, -0.16 to 0.45), 0.19 (95% CI, -0.19 to 0.49), and 0.26 (95% CI, -0.14 to 0.54) on days 1, 2, 3, and 4 of hospitalization, respectively. In the OHCA patients without malignant BBB disruption, correlation coefficients between Qa and ICP were 0.55 (95% CI, -0.39 to 1.00), -0.05 (95% CI, -0.41 to 1.00), 0.68 (95% CI, -0.15 to 0.40), and 0.43 (95% CI, -0.56 to 1.00) on days 1, 2, 3, and 4 of hospitalization, respectively. In the OHCA patients with malignant BBB disruption, correlation coefficients between Qa and ICP were -0.22 (95% CI, -0.54 to 0.21), -0.01 (95% CI, -0.37 to 0.34), -0.05 (95% CI, -0.44 to 0.37), and 0.07 (95% CI, -0.51 to 0.59) on days 1, 2, 3, and 4 of hospitalization, respectively.
Comparison of ICP between the patients with and without malignant BBB disruption
On days 1 and 2 of hospitalization, ICP was higher in the patients with malignant BBB disruption than in the patients without malignant BBB disruption, however, on days 3 and 4 of hospitalization, ICP was similar in both patient groups (Table 2 and Fig. 2).

Intracranial pressure (ICP) over time in out-of-hospital cardiac arrest patients with and without malignant blood-brain barrier (BBB) disruption on days 1, 2, 3, and 4 of hospitalization. On days 1 and 2 of hospitalization, significant changes were observed in ICP in the patients with malignant BBB disruption; however, significant changes were observed in ICP between days 1, 2, and 3 of hospitalization in the patients without malignant BBB disruption. In addition, ICP was higher in the patients with malignant BBB disruption than in the patients without malignant BBB disruption on days 1 and 2 of hospitalization. *P<0.05; ***P<0.001.
Comparison of daily ICP changes in the OHCA patients with and without malignant BBB disruption
In the patients without malignant BBB disruption, significant changes were observed in ICP between days 1, 2, and 3 of hospitalization. In the patients with malignant BBB disruption, significant changes were observed in ICP between days 1 and 2 of hospitalization (Table 3).
DISCUSSION
In the present study, differences were observed in the incidence of shockable rhythm at the time of CA, low flow time, and GCS immediately after ROSC between the OHCA patients with and without malignant BBB disruption. ICP in the patients with malignant BBB disruption was significantly higher than in the patients without malignant BBB disruption on days 1 and 2 of hospitalization, although the average ICP in OHCA patients treated with TTM was between 9.58 and 16.10 mmHg at any time point in both patient groups. A different pattern was observed with a statistically significant increase in ICP until day 2 of hospitalization in the patients with malignant BBB disruption; however, an increase in ICP in the patients without malignant BBB disruption was not observed until day 3 of hospitalization, and the ICP in both patient groups became similar after day 3 of hospitalization.
The BBB plays an important role in maintaining optimal brain function by regulating the interstitial fluid microenvironment. BBB disruption leads to its failure to sufficiently block the transport of neurotoxins to the central nervous system, resulting in cerebral edema [26-28]. When moderate or severe BBB disruption occurs, the probability of poor neurological outcomes is higher than good neurological outcomes [18]. To evaluate the degree of BBB disruption, several methods have been used, such as dynamic contrast-enhanced magnetic resonance imaging, S100B protein, and Qa [29,30].
Among these methods, Qa is a reliable method for the functional assessment of BBB disruption and commonly used in routine clinical practice and research [31,32]. In particular, the BBB disruption score using Qa is a valuable predictor of neurological prognosis in OHCA patients treated with TTM, and the area under the receiver operating characteristic curve of BBB disruption score cutoff value of 9 for poor neurological outcomes was 0.94 [18]. In the present study, a BBB disruption score cutoff value ≥9 was defined as malignant BBB disruption, and 80.5% of OHCA patients with malignant BBB disruption had poor neurological outcomes.
The disruption of the BBB under ischemic conditions is multifactorial and may involve factors such as enhanced production of inflammatory cytokines, excessive oxidative stress, and upregulation of vascular endothelial growth factors [33,34]. An increase in BBB permeability allows extravasation of albumin and other high molecular weight compounds into the extracellular compartment of the brain, resulting in vasogenic edema and subsequent cell injury. Increase in BBB permeability and formation of cerebral edema may lead to increased ICP [35-37]. However, in a previous study, ICP was within the normal range in both patient groups, although the ICP in the poor neurological outcome group was higher than in the good neurological outcome group [21].
TTM reduces reperfusion injury by modulating the following mechanisms: production of free oxygen radicals, excitotoxic neurotransmitter release, and calcium influx. In addition, TTM reduces the cerebral metabolic rate and prevents mitochondrial breakdown and cellular apoptosis. Finally, TTM lowers the ICP by preventing BBB disruption and reducing brain edema [38,39]. In the present study, ICP in OHCA patients treated with TTM was within the normal range, and statistically significant correlations were not found between Qa and ICP in both OHCA patients with and without malignant BBB disruption, although ICP in the subjects with malignant BBB disruption was higher than in the patients without malignant BBB disruption on days 1 and 2 of hospitalization.
The present study had several limitations. First, this was a single-center study with a small sample size, which might limit the generalizability of our findings. Second, CSF albumin levels were measured in CSF obtained using a lumbar catheter. In a previous study, the albumin concentration in the lumbar space was reportedly 2.2 times higher than in the ventricle [40]. Third, other biomarkers, such as neurofilament light chain, were not measured; therefore, we could not speculate on the changes in these parameters. Finally, the investigator was not blinded throughout the experiment. Future studies involving blinding are needed to address this limitation.
In conclusion, among OHCA patients treated with TTM, ICP was higher on days 1 and 2 of hospitalization and the increase in ICP occurred earlier in OHCA patients with malignant BBB disruption than in OHCA patients without malignant BBB disruption. However, the average ICP ranged from 9.58 to 16.10 mmHg at any time point in both patient groups.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
References
Article information Continued
Notes
Capsule Summary
What is already known
Ischemia-reperfusion brain injury following out-of-hospital cardiac arrest (OHCA) due to oxidative stress, intracellular calcium influx, and glutamate production cause blood-brain barrier (BBB) disruption, resulting in brain edema and increased intracranial pressure (ICP).
What is new in the current study
Although ICP was within the normal range following OHCA regardless of malignant BBB disruption, the increase in ICP in the OHCA patients with malignant BBB disruption was higher and earlier than in OHCA patients without malignant BBB disruption.