The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study
Article information
Abstract
Objective
To describe the effect of liver disease (LD) on lactate clearance during early sepsis resuscitation.
Methods
This is a multicenter randomized clinical trial. An initial lactate >2 mmol/L and subsequent serum lactate measurement within 6 hours were required for inclusion. LD was categorized by two methods: 1) past medical history (PMH) categorized as no LD, mild LD (no Child’s score criteria, but PMH of hepatitis B/C), cirrhosis; and 2) measurable liver dysfunction determined by the liver component of the sequential organ failure assessment (L-SOFA) score as no dysfunction (L-SOFA score 0), mild dysfunction (score 1), moderate-severe dysfunction (score 2 to 4). Primary outcome was the rate of lactate normalization.
Results
One hundred eighty-seven patients were included. When categorized by PMH, 169 patients had no LD, 6 mild LD, and 12 cirrhosis. 63/169 (37%) of patients with no LD achieved lactate normalization, compared to 4/6 (67%) with mild LD, and 1/12 (8%) with cirrhosis (P<0.03). Categorized by L-SOFA score, 59/124 (47%) patients with L-SOFA 0 achieved lactate normalization, compared to 6/31 (19%) with L-SOFA 1, and 3/32 (9%) with L-SOFA 2–4 (P<0.01). Relative lactate clearance [(initial lactate–subsequent lactate)/initial lactate] was lower in patients with more advanced LD by PMH (37.7 vs. 40.4 vs. 21.8, P=0.07), and lower with increasing L-SOFA score (42.0 vs. 30.1 vs. 23.4, P=0.01).
Conclusion
Liver dysfunction was significantly associated with impaired lactate clearance and normalization during the early resuscitation of sepsis.
INTRODUCTION
Elevated serum lactate has previously been shown to be a poor prognostic marker, associated with both increased morbidity and mortality in sepsis [1-3]. Additionally, previous reports have demonstrated improved outcomes in patients with severe sepsis and septic shock who achieve both lactate clearance and lactate normalization [4-10]. The 2012 Surviving Sepsis Campaign Guidelines recommend lactate normalization as a target of resuscitation in patients with an elevated lactate level, with a goal to achieve normalization as rapidly as possible [11].
Although research continues to suggest that the development of hyperlactatemia is a complex, multifactorial, and potentially patient-specific process, it is accepted that hyperlactatemia can result from either overproduction, impaired clearance, or a combination of the two. Prior research has shown that some patients with hypotension and shock develop hyperlactatemia, while others do not [12-15], suggesting a more complex process than simple hypoperfusion or hypoxia. Lactate is metabolized primarily by the liver [16-18]. Though previous studies have suggested that liver dysfunction is associated with higher lactate levels in the acutely ill [15,19,20], the impact of liver disease (LD) on the early stages of an acute resuscitation in sepsis is not well-known. The objective of this study was to describe the effects of LD on lactate kinetics during early resuscitations in severe sepsis and septic shock.
METHODS
Study design
We conducted a secondary analysis of a completed, large, multicenter randomized control trial evaluating the non-inferiority of lactate clearance versus central oxygen saturation (ScvO2) as a marker of adequate oxygen delivery during early quantitative resuscitations in septic patients [21]. The methodology of the trial has been previously reported [21]. Briefly, the trial was conducted at large, urban medical centers between January 2007 and January 2009. The institutional review board at each institution approved the study, with all participants or surrogates providing written informed consent. The trial was registered on Clinicatrials.gov, identifier NCT00372502. Abbreviated inclusion criteria were adults with suspected infection, two or more systemic inflammatory response criteria, and systolic blood pressure <90 mmHg after a 20 mL/kg fluid challenge or an initial lactate >4 mmol/L [21].
In that study, patients were randomized to 1 of 2 study groups. Each group had a structured quantitative resuscitation protocol, which has been previously described and published [21]. The ScvO2 group (n=150) was resuscitated by directing therapy to meet threshold values of central venous pressure, mean arterial pressure, and ScvO2. The lactate clearance group (n=150) had similar goals in central venous pressure, mean arterial pressure, and then lactate clearance (decrease in lactate of at least 10% over at least 2 hours) instead of ScvO2. Protocols were followed until all endpoints were met or a maximum time of 6 hours elapsed [21].
Data analysis
Given our objective of evaluating lactate clearance, in the present study we included only patients that had an initially elevated lactate level (≥2 mmol/L) and a subsequent lactate level measured within six hours. We further categorized these patients by the presence of LD, as defined in two ways. In the first classification, patients were categorized by their reported past medical history (PMH) of LD, defined as either: no LD, mild LD (no Child’s score criteria [22,23], but a PMH of hepatitis B or C), or cirrhosis. For the second classification, patients were categorized by measurable liver dysfunction using the liver component of the sequential organ failure assessment (L-SOFA) score at enrollment, defined as either: no dysfunction (L-SOFA 0), mild dysfunction (L-SOFA 1), or moderate-severe dysfunction (L-SOFA 2–4). As described by Vincent and colleagues, the L-SOFA score is calculated by serum bilirubin values as follows: L-SOFA 0, <1.2 mg/dL; L-SOFA 1, 1.2 to 1.9 mg/dL; L-SOFA 2, 2.0 to 5.9 mg/dL; L-SOFA 3, 6.0 to 11.9 mg/dL; L-SOFA 4, >12.0 [24,25].
The primary outcome of this study was the difference in the rate of lactate normalization (initial elevated lactate with all subsequent measurements <2 mmol/L) between groups as defined by either PMH or L-SOFA score. The secondary outcome was the difference in relative lactate clearance [(initial lactate–subsequent lactate)/initial lactate] between groups. Fischer’s exact, Mann-Whitney U, and Kruskal-Wallis tests were used as appropriate. All statistical tests were two sided with P<0.05 considered significant. Data were analyzed using STATA ver. 10.0 (Stata Corp., College Station, TX, USA) or StatsDirect ver. 2.7.7 (StatsDirect, Cheshire, England).
RESULTS
A total of 187 patients had an initial lactate ≥2 mmol/L and a subsequent lactate level measured within 6 hours and were therefore included in the present analysis, and represents the same cohort used in previous work by our group to compare the prognostic value of various measures of lactate clearance [8]. One hundred thirteen patients were excluded from the original study for either lack of an initial lactate measurement (96 patients) or repeat lactate level within 6 hours (17 patients) (Fig. 1). When compared to the included patients, the 113 excluded had no significant differences in age, sex, or race; however, the excluded patients were less likely to have a PMH of diabetes mellitus and hypertension, and had a lower total SOFA score than those patients who were included in this analysis. No significant difference in mortality was noted between the excluded and included patients. When categorized by PMH, 169 patients had no LD, 6 patients had mild LD, and 12 patients had cirrhosis. With the exception of age, no significant differences were noted in demographics, comorbid conditions, and specifically renal function, source of infection, or overall in-hospital mortality between the three groups (Table 1). Of note, while not statistically significant, baseline lactate levels were higher in the cirrhosis group overall. The median time of repeat lactate measurement for the patients with no LD, mild LD, and cirrhosis was 135 minutes (interquartile range [IQR], 112 to 205), 153 minutes (IQR, 125 to 313), and 121 minutes (IQR, 117 to 153), respectively. No statistical difference was found between the groups (P<0.53).
For the primary outcome of lactate normalization, 63/169 (37%; 95% CI, 30% to 45%) of patients with no LD achieved lactate normalization, compared to 4/6 (67%; 95% CI, 12% to 100%) of those with mild LD, and 1/12 (8%; 95% CI, -10% to 27%) of those with cirrhosis (P<0.03). Post hoc analysis revealed differences in the no LD group compared to cirrhosis group (37% and 8%, respectively, P=0.04) as well as the mild LD (67%) compared with cirrhosis (8%, P=0.01) but no differences in the no LD and mild LD groups (Fig. 2). The secondary outcome, relative lactate clearance, was likewise lower in patients with more advanced LD by PMH (37.7 vs. 40.4 vs. 21.8, P=0.07), with significant differences between no LD (37.7%) compared with cirrhosis (21.8%, P=0.03) (Fig. 2).
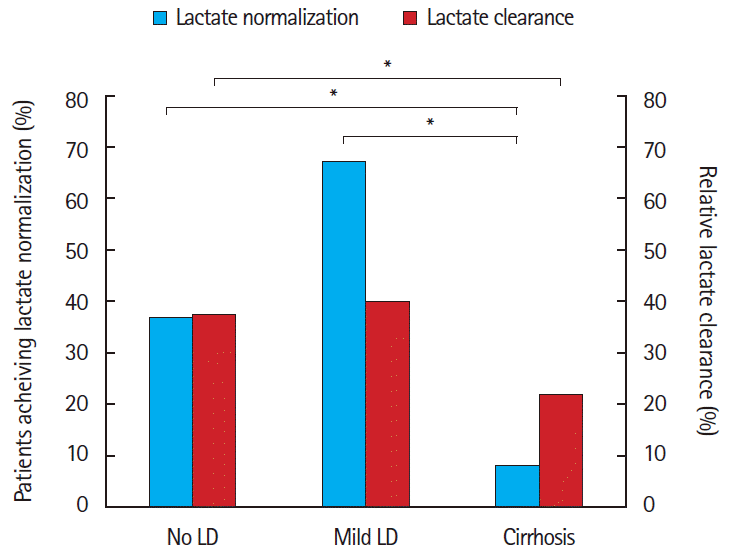
Achievement of lactate normalization and rate of lactate clearance when classified by past medical history of liver disease (LD). *P<0.05.
When categorized by measureable liver dysfunction by L-SOFA, 124 patients had an L-SOFA score of 0, 31 had a score of 1, and 32 had a score of 2 to 4. No significant differences were noted in patient demographics, comorbidities, and specifically renal function, or overall in-hospital mortality between the groups; however, significant differences were noted between the groups in total SOFA score and initial lactate level, as well as in the source of infection (Table 2). Of note, while not statistically significant, baseline lactate levels were higher in the L-SOFA 2–4 groups overall. The median time of repeat lactate measurement for the L-SOFA 0, L-SOFA 1, and L-SOFA 2–4 was 134 minutes (IQR, 114 to 216), 148 minutes (IQR, 123 to 205), and 126 minutes (IQR, 114 to 153), respectively. No statistical difference was found between the groups (P<0.35).
For the primary outcome, 59/124 (47%; 95% CI, 37% to 56%) patients with a L-SOFA of 0 achieved lactate normalization, compared to 6/31 (19%; 95% CI, 5% to 34%) with L-SOFA of 1, and 3/32 (9%; 95% CI, -1% to 20%) with a L-SOFA of 2–4 (P<0.01). Post hoc analysis revealed differences in L-SOFA 0 group (47%) as compared to the L-SOFA 1 group (19%, P<0.01), and between the L-SOFA 0 group compared with the L-SOFA 2–4 group (47% and 9%, respectively, P<0.01), but no differences in the L-SOFA 1 compared to L-SOFA 2–4 groups (Fig. 3). For the secondary outcome, lactate clearance percent decreased with increasing L-SOFA score (42.0 vs. 30.1 vs. 23.4, P=0.01). Secondary analysis showed a significant difference in relative lactate clearance between the group with L-SOFA 0 (42%) compared to L-SOFA 2–4 (23.4%, P<0.01) (Fig. 3).
DISCUSSION
In this analysis, we sought to evaluate the effect of LD on lactate clearance during the early phase of quantitative resuscitations in severe sepsis and septic shock. Our results indicate that liver dysfunction, defined either by preexisting PMH or by L-SOFA score, are associated with differences in both lactate clearance and lactate normalization. These results highlight a patient population in whom delayed lactate clearance, and thus prolonged hyperlactatemia may occur, and a clinical scenario where the resolution of hyperlactatemia may be more difficult to achieve.
For this analysis, we chose to evaluate the presence of LD in two distinct ways, by reported PMH of LD and by measurable liver dysfunction using the liver component of their SOFA score on presentation. Using both of these approaches, we were able to capture patients who historically had known chronic disease, but potentially no laboratory changes, and patients who may or may not have known of underlying liver dysfunction, but acutely had measurable objective evidence of liver dysfunction on presentation. Using two approaches, we were able to more fully assess the impact of LD and dysfunction on lactate kinetics, using easily attainable, clinically relevant definitions. The fact that outcomes were similar regardless of the method of categorization supports the conclusion that the observed associations are likely important and clinically relevant.
While the difference in lactate normalization was significant in both the group defined by PMH and L-SOFA, a more obvious association was noted when using L-SOFA score. While this may indicate that objective evidence of liver dysfunction by laboratory testing may be more important than PMH in predicting impact on lactate clearance, this may also simply be a reflection of the fact that patients were more evenly distributed across LD categories as defined by SOFA score as compared to PMH. This is particularly evident in the case of “mild” groups. Using the L-SOFA definition (liver SOFA 1), there were observable effects on lactate normalization, but, the historical presence of “mild LD” did not appear to impact lactate normalization or the rate of lactate clearance in this study, though this may have been limited by the small sample size (6 patients) and lower comorbidity burden (Table 1) within this subgroup. The presence of cirrhosis by history, however, had marked effects on both lactate normalization and clearance. These results suggest that acute liver dysfunction on presentation, assessed using the L-SOFA, or a PMH of cirrhosis may impact the early resuscitation goals in severe sepsis and septic shock. Despite the notable effects of LD on lactate normalization and rate of clearance, this did not translate into a statistically significant difference in mortality between the groups, although the highest mortality rates were observed in the patients with either cirrhosis or L-SOFA 2–4. However, given that mortality was not a primary outcome in our study and noting the relatively small number of patients with severe LD, these observations are merely hypothesis generating.
Our results are similar to previous studies evaluating lactate levels in patients with LD. De Jonghe et al. [19] noted an elevated lactate level in patients with evidence of early hepatic dysfunction and circulatory failure. While they found hepatic dysfunction was associated with an elevated serum lactate, they did not note a difference in mortality. However, contrasting with our study, their study evaluated intensive care unit patients with undifferentiated circulatory failure and was not specific to severe sepsis or septic shock, nor did it assess lactate clearance [19]. Our study extends their findings by additionally evaluating lactate normalization and clearance during acute, quantitative resuscitations in the initial resuscitation period of patients with severe sepsis and septic shock. In another study, Dugas et al. [15] evaluated vasopressor dependent patients with septic shock and found that acute liver injury and history of LD were associated with elevated lactate levels. While they concluded that hyperlactatemia as a sole end point of resuscitation may be inadequate [15], our findings suggest a subgroup of septic patients where clinicians may be able to anticipate impaired lactate clearance and/or normalization, and could consider this during their resuscitative efforts.
Our study does have several other important limitations. First, this is a secondary analysis with the inherent limitations of that methodology. Second, as previously mentioned, the number of patients with mild and moderate to severe LD or dysfunction was relatively small, making generalizable conclusion difficult to fully assess. Finally, in the original study, patients were divided into 1 of 2 treatment groups with different treatment protocols [21]; however, no significant difference in treatment groups was noted between the groups in our study, the treatment protocols had similar targets and goals [21], and no significant differences were noted between the groups in the volume of intravenous fluids given in the first six hours, nor in the use of vasopressors (Tables 1, 2), all suggest that it is unlikely that this affected the outcome of our present study. While the patients excluded from the original study had less comorbid conditions and lower severity of illness on admission than included patients, given that inclusion criteria for this study required an elevated serum lactate level, these results are not unexpected and are consistent with prior literature [1,12]. Thus, we think it likely did not affect our results as we were comparing only patients with hyperlactatemia, who did or did not have LD.
In this analysis, liver dysfunction, as defined by either PMH or by objective laboratory values at presentation, was significantly associated with impaired lactate clearance and normalization during the early resuscitation of severe sepsis and septic shock. Clinicians should consider these findings during management of sepsis patients with LD. Further studies are necessary to determine if alternative lactate clearance resuscitation goals are necessary in this subgroup of patients.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Although research has shown that the liver is the primary organ responsible for lactate clearance and that lactate clearance and normalization are associated with improved outcomes in severe sepsis and septic shock, to our knowledge, there has been no examination of the impact of liver dysfunction on lactate clearance and normalization in early resuscitations in sepsis.
What is new in the current study
This study evaluates the effect of liver disease on lactate clearance and normalization during early resuscitations in severe sepsis and septic shock. Our results indicate that liver dysfunction does impact lactate clearance and normalization, which could impact clinicians performing resuscitations of septic patients with liver disease.