AbstractObjectiveTo examine the association of inferior vena cava (IVC) diameter ratio measured using computed tomography with outcomes in patients with gastrointestinal bleeding (GIB).
MethodsA single-center retrospective observational study was conducted on consecutive patients with GIB who presented to the emergency department. The IVC diameter ratio was calculated by dividing the maximum transverse and anteroposterior diameters perpendicular to it. The association of the IVC diameter ratio with outcomes was examined using multivariable logistic regression analysis. The primary outcome was in-hospital mortality. The area under the receiver operator characteristic curve (AUC) of the IVC diameter ratio was calculated, and the sensitivity and specificity, including the cutoff values, were computed.
ResultsIn total, 585 patients were included in the final analysis. The in-hospital mortality rate was 4.6% (n=27). The IVC diameter ratio was significantly associated with higher in-hospital mortality in multivariable logistic regression analysis (odds ratio, 1.793; 95% confidence interval [CI], 1.239–2.597; P=0.002). The AUC of the IVC diameter ratio for in-hospital mortality was 0.616 (95% CI, 0.498–0.735). With a cutoff of the IVC diameter ratio (≥2.1), the sensitivity and specificity for predicting in-hospital mortality were 44% (95% CI, 26%–65%) and 71% (95% CI, 67%–75%), respectively.
INTRODUCTIONAcute gastrointestinal bleeding (GIB) is a common presentation in the emergency department (ED), with a prevalence of approximately 45 to 172 per 100,000 individuals per year [1,2]. Data from the UK continue to show a mortality rate of 7% to 14%, imposing a significant burden on the healthcare system [3-5]. The technical developments in endoscopic hemostasis and the continued use of proton pump inhibitors are thought to have influenced the epidemiology and outcomes of peptic ulcer bleeding. Nevertheless, more than 60 of 100,000 patients are hospitalized for GIB in the US per year, with a remarkable mortality rate of 8% to 10%, and the cost associated with hospitalization is more than 100,000 US dollars per year [1,6,7].
The severity of GIB varies from mild symptoms to death. Therefore, risk assessment is recommended in patients with GIB to predict outcomes, such as death and re-bleeding, and the need for clinical interventions, such as endoscopic hemostasis, transfusion, or radiologic intervention. Risk assessment is also necessary to determine the treatment level (inpatient vs. outpatient or general ward vs. intensive care unit) [8]. There are many scores for risk stratification in patients with GIB. Among the various risk assessment scores, the Glasgow Blatchford score (GBS) and AIMS65 score (albumin <30 g/L [A], International normalized ratio >1.5 [I], altered mental state [M], systolic blood pressure ≤90 [S], and age >65 years [65]), which can be calculated without the need for endoscopy, are widely used [9,10]. However, the use of these scores for clinical decision-making is limited in the ED. Since GBS includes the presence of an underlying disease, it is problematic since definitions are subjective. The AIMS65 score effectively predicts mortality, but its predictive validity for other outcomes, such as the need for endoscopic hemostasis or blood transfusion, has not been established [11-14]. According to previous studies, inferior vena cava (IVC) collapsibility and diameter are known factors that can predict volume status [15]. In trauma or sepsis patients, the association between IVC diameter measured on computed tomography (CT) and patient prognosis has been reported [16,17].
However, no study has addressed the predictive value of IVC diameter ratio in patients with GIB. CT is often performed in patients who visit the ED due to GIB. In a recent study, CT was recommended as a suitable modality to identify the source of acute GIB [18-20]. It can also be used to rapidly diagnose active bleeding. Therefore, considering these findings, the present study aimed to determine whether the IVC diameter measured on CT can help evaluate the prognosis of patients.
METHODSEthical statementsThis study was approved by the institutional review board of Hanyang University Hospital (No. HYUH 2020-11-010-004), and the need to obtain informed consent was waived due to the nature of the observational study. This study protocol was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines.
Study design and populationThis was a retrospective observational cohort study of patients with GIB conducted between January 2016 and June 2020. The study included adults aged >18 years who visited the ED of a university-affiliated hospital in Seoul, Korea. We extracted the data of patients with GIB through a chart review. GIB was diagnosed when a patient visited the ED with one of the following chief complaints: hematemesis, melena, and hematochezia. The exclusion criteria were as follows: direct transfer to other hospitals from the ED, no CT examination, and patients who had signed a “do not attempt resuscitation order.”
Definitions and outcomesCT was performed by the treating physician on patients with GIB. Contrast-enhanced or non-contrast-enhanced CT was performed with an interval of 3 mm. The IVC diameter was measured using CT scans at a level just below the renal vein, which is the area least affected by patient breathing (Fig. 1) [21].
After assessing the maximum transverse diameter of the IVC, the anteroposterior diameter perpendicular to the transverse diameter was measured by an emergency medicine chief resident who was blinded to patient outcomes. IVC diameter ratio was calculated as the maximal transverse diameter divided by anteroposterior diameter. The primary outcome of this study was in-hospital mortality. Secondary outcomes included endoscopic hemostasis, red blood cell transfusion, and radiologic embolization. Composite outcomes included in-hospital mortality and all secondary outcomes.
Statistical analysisThe study data were reported as mean±standard deviation or median with an interquartile range for continuous variables, as appropriate. Student t-test or Mann-Whitney U-test was used to compare continuous variables. The chi-square test or Fisher exact test was used to compare categorical variables. Logistic regression analysis was performed to assess the independent effect of IVC diameter ratio on in-hospital mortality, after adjusting for pre-defined confounding variables such as age, gender, GBS, AIMS65 score, systolic blood pressure (SBP), heart rate, heart failure, hypertension, diabetes mellitus, and liver cirrhosis. The area under the curve (AUC) was computed to examine the prognostic value of the IVC diameter ratio in predicting in-hospital mortality. Optimal threshold values were determined by maximizing the Youden index [22]. The sensitivity, specificity, positive predictive value, and negative predictive value of the IVC diameter ratio were calculated. A two-sided P-value of 0.05 was considered significant. All statistical analyses were performed using PASW Statistics ver. 18 (SPSS Inc., Chicago, IL, USA).
RESULTSParticipant characteristicsIn total, 1,102 patients were screened by chart review from January 2016 to June 2020. Among these, 473 patients who did not undergo CT (Fig. 2), 14 patients who were transferred from the ED to another hospital, and 30 patients with “do not attempt resuscitation order” were excluded. Finally, 585 patients were included in the analysis. The mean age of the patients was 62.5 years, and 65.0% of the patients were male. The in-hospital mortality rate was 4.6% (n=27). Further, 80.5% of the patients showed a composite outcome (n=471). The median GBS and AIMS65 score were 8 and 1, respectively (Table 1).
Multivariable logistic regression analysis of factors predicting in-hospital mortalityUnivariate logistic regression analysis of factors predicting inhospital mortality was conducted in Supplementary Table 1. Multivariate logistic regression analysis was performed to examine the association between IVC diameter ratio and in-hospital mortality in patients with GIB (Table 2). The adjusted odds ratio (aOR) of the IVC diameter ratio for in-hospital mortality was 1.793 (95 % confidence interval [CI], 1.239–2.597), and it was statistically significant (P=0.002). Age, systolic blood pressure, heart rate, and heart failure were independently associated with in-hospital mortality (aOR, 1.032, 0.977, 1.021, and 13.988, respectively).
The AUC of the IVC diameter ratio for predicting in-hospital mortality was 0.616 (95% CI, 0.498–0.735) (Fig. 3). In addition, the AUCs of the IVC diameter ratio for predicting composite outcomes such as endoscopic hemostasis, red blood cell transfusion, radiologic embolization, and intensive care unit admission were 0.520 (95% CI, 0.458–0.582), 0.531 (95% CI, 0.476–0.587), 0.518 (95% CI, 0.379–0.656), and 0.534 (95% CI, 0.472–0.597), respectively (Supplementary Figs. 1-5). The cutoff value of the IVC diameter ratio for predicting in-hospital mortality, maximizing the sum of sensitivity and specificity, was 2.1. The overall diagnostic accuracy of the cutoff value of the IVC diameter ratio (≥2.1) was 70% (95% CI, 65%-74%). The sensitivity, specificity, positive predictive value, and negative predictive value of the cutoff values were 44% (95% CI, 26%–65%), 71% (95% CI, 67%–75%), 8% (95% CI, 5%–12%), and 96% (95% CI, 94%–97%), respectively.
DISCUSSIONThe results of this study indicate that a high IVC diameter ratio measured by CT in patients with GIB was associated with poor outcomes. However, the AUC of the IVC diameter ratio was low (0.616), and the diagnostic accuracy of the optimal cutoff value was only 70%. Therefore, further studies should be conducted to validate the usefulness of IVC diameter ratio as a prognostic factor in patients with GIB.
To the best of our knowledge, the present study is the first to evaluate the association between the IVC diameter ratio measured on CT scans and in-hospital mortality in patients with GIB who visit the ED. Previous studies have investigated the association between IVC collapsibility or diameter, but not the IVC diameter ratio, and the outcomes of critically ill patients with various diseases. The strengths of this study include the inclusion of patients with GIB who underwent uniform treatment in a single institution. Unlike previous studies that used ultrasound, the diameter of IVC was measured more objectively using CT in the present study.
Numerous tools/scores have been used to evaluate the prognosis of patients with GIB. Although there are many scoring systems, the GBS and AIMS65 scores have been well validated in many studies on GIB [23,24]. However, these scoring systems have limitations in identifying high-risk patients who may require inpatient endoscopy, embolization, or surgical treatment, as well as identifying patients at high risk of mortality, especially among those with GBS [24-27]. Moreover, the subjectivity of the definitions of hepatic disease and cardiac disease included in the GBS makes its application in clinical practice challenging [28]. A multicenter study revealed that a GBS of ≤1 appeared to be the optimum threshold for directing patients to outpatient management without endoscopy [14]. Although assessment using GBS can accurately identify low-risk patients suitable for early discharge, it cannot unequivocally identify individual patients who require intensive monitoring or are at risk of death [29,30]. In addition, GBS was inferior to the AIMS65 score in predicting inpatient mortality due to upper GIB, despite being superior in predicting blood transfusion [24]. The AIMS65 score was superior to the GBS in predicting mortality, but its predictive value for endoscopic hemostasis was inferior [14]. Furthermore, the use of AIMS65 score to identify patients at very low risk of re-bleeding or mortality is not recommended [8]. The AIMS65 score was designed to be used with high cutoff values to identify patients at high risk of mortality rather than to identify those at low risk with safe discharge.
Previous studies on patients with trauma or septic shock have reported that the IVC diameter predicts hypovolemia, blood loss, and in-hospital mortality [31-33]. They have also reported that the IVC diameter ratio was significantly correlated with markers of shock and was a predictor of mortality in patients with trauma and septic shock [16,34]. A retrospective cohort study reported that the IVC diameter ratio was significantly correlated with other known markers of shock and was an independent predictor of mortality in severely injured trauma patients [16]. In their study, the cutoff value for the IVC diameter ratio was defined as ≥1.9. They performed an AUC analysis to maximize the sensitivity and specificity of the IVC diameter ratio to predict mortality and arrived at a ratio of 1.9. In our study, the cutoff value for the IVC diameter ratio, maximizing both sensitivity and specificity, was 2.1. This is not significantly different from the cutoff value of 1.9 noted in trauma patients, and blood loss appears to be the main mechanism that causes shock in patients with GIB and trauma.
Ultrasonography is useful for measuring IVC diameter or changes in IVC diameter caused by respiration. However, the use of ultrasound has some drawbacks: there is variability in the results depending on the operator’s skill, and accurate measurement is difficult when the patient is obese or has air in the bowel. CT is useful for quickly determining the bleeding site or active bleeding site in patients with GIB [13,15,16]. By addressing the association of the IVC diameter ratio assessed using CT with patient outcomes, our study might help in the rapid prognostic evaluation of patients with GIB who visit the ED.
This study has several limitations. First, 473 patients were excluded because they did not undergo CT, and the results of this study have a selection bias, making it difficult to extrapolate these results to the entire GIB population. A prospective study is needed to analyze the results of the IVC diameter ratio measurements using CT in all patients with GIB. Second, it is difficult to rule out selection bias in a single-center retrospective study. Third, the amount of fluid supplied before CT was not adjusted. Fourth, it was not specified whether positive pressure mechanical ventilation was performed during CT, which could have affected the diameter of IVC. However, because only six patients underwent mechanical ventilation, it did not appear to have affected the main results. Fifth, the IVC diameter ratio did not show superiority in diagnostic performance over the GBS and AIMS65 scores. However, simple measurement of the IVC diameter ratio seems to be a strength in that there is no significant difference in predictive power when compared to the complex scoring system. Finally, IVC diameter was measured by a single investigator, and the measured value was not compared with that of another investigator. Therefore, the possibility of bias in IVC diameter measurements cannot be ruled out.
In conclusion, the IVC diameter ratio measured on CT scans was independently associated with in-hospital mortality in patients with GIB. However, the AUC of the IVC diameter ratio for in-hospital mortality was low. Further prospective studies are needed to determine the prognostic value of the IVC diameter ratio.
Supplementary MaterialsSupplementary Fig. 1.Comparison of the inferior vena cava (IVC) diameter ratio with the Glasgow Blatchford score (GBS) and AIMS65 score for predicting composite outcomes. Supplementary Fig. 2.Comparison of the inferior vena cava (IVC) diameter ratio with the Glasgow Blatchford score (GBS) and AIMS65 score for predicting endoscopic hemostasis. Supplementary Fig. 3.Comparison of the inferior vena cava (IVC) diameter ratio with the Glasgow Blatchford score (GBS) and AIMS65 score for predicting red blood cell transfusion. Supplementary Fig. 4.Comparison of the inferior vena cava (IVC) diameter ratio with the Glasgow Blatchford score (GBS) and AIMS65 score for predicting radiologic embolization. Supplementary Fig. 5.Comparison of the inferior vena cava (IVC) diameter ratio with the Glasgow Blatchford score (GBS) and AIMS65 score for predicting intensive care unit admission. Supplementary Table 1.Univariate logistic regression analysis of factors predicting in-hospital mortality Supplementary materials are available from: https://doi.org/10.15441/ceem.21.099.
ACKNOWLEDGMENTSThis research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Korean government (No. NRF-2019M3E5D1A01066060).
REFERENCES1. Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci 2018; 63:1286-93.
2. Lanas A, Garcia-Rodriguez LA, Polo-Tomas M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol 2009; 104:1633-41.
3. Abougergi MS, Travis AC, Saltzman JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc 2015; 81:882-8.
4. Loperfido S, Baldo V, Piovesana E, et al. Changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc 2009; 70:212-24.
5. Theocharis GJ, Thomopoulos KC, Sakellaropoulos G, Katsakoulis E, Nikolopoulou V. Changing trends in the epidemiology and clinical outcome of acute upper gastrointestinal bleeding in a defined geographical area in Greece. J Clin Gastroenterol 2008; 42:128-33.
6. Quan S, Frolkis A, Milne K, et al. Upper-gastrointestinal bleeding secondary to peptic ulcer disease: incidence and outcomes. World J Gastroenterol 2014; 20:17568-77.
7. Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011; 60:1327-35.
8. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the International Consensus Group. Ann Intern Med 2019; 171:805-22.
9. Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993- 2002: a population-based cohort study. Am J Gastroenterol 2006; 101:945-53.
10. El-Tawil AM. Trends on gastrointestinal bleeding and mortality: where are we standing? World J Gastroenterol 2012; 18:1154-8.
11. Robertson M, Majumdar A, Boyapati R, et al. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems. Gastrointest Endosc 2016; 83:1151-60.
12. Budimir I, Gradiser M, Nikolic M, et al. Glasgow Blatchford, pre-endoscopic Rockall and AIMS65 scores show no difference in predicting rebleeding rate and mortality in variceal bleeding. Scand J Gastroenterol 2016; 51:1375-9.
13. Kim MS, Choi J, Shin WC. AIMS65 scoring system is comparable to Glasgow-Blatchford score or Rockall score for prediction of clinical outcomes for non-variceal upper gastrointestinal bleeding. BMC Gastroenterol 2019; 19:136.
14. Stanley AJ, Laine L, Dalton HR, et al. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ 2017; 356:i6432.
15. Dipti A, Soucy Z, Surana A, Chandra S. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. Am J Emerg Med 2012; 30:1414-9.
16. Johnson JJ, Garwe T, Albrecht RM, et al. Initial inferior vena cava diameter on computed tomographic scan independently predicts mortality in severely injured trauma patients. J Trauma Acute Care Surg 2013; 74:741-5.
17. Kim JH, Kim WY, Oh J, Kang H, Lim TH, Ko BS. Association of inferior vena cava diameter ratio measured on computed tomography scans with the outcome of patients with septic shock. Medicine (Baltimore) 2020; 99:e22880.
18. Lee CM, Jang JK, Shin JH, Song SY, Kang BK. Role of computed tomography angiography for acute gastrointestinal bleeding. Gastrointest Interv 2018; 7:106-11.
19. Wells ML, Hansel SL, Bruining DH, et al. CT for evaluation of acute gastrointestinal bleeding. Radiographics 2018; 38:1089-107.
20. Wortman JR, Landman W, Fulwadhva UP, Viscomi SG, Sodickson AD. CT angiography for acute gastrointestinal bleeding: what the radiologist needs to know. Br J Radiol 2017; 90:20170076.
21. Wallace DJ, Allison M, Stone MB. Inferior vena cava percentage collapse during respiration is affected by the sampling location: an ultrasound study in healthy volunteers. Acad Emerg Med 2010; 17:96-9.
22. Perkins NJ, Schisterman EF. The Youden Index and the optimal cut-point corrected for measurement error. Biom J 2005; 47:428-41.
23. Laursen SB, Hansen JM, Schaffalitzky de Muckadell OB. The Glasgow Blatchford score is the most accurate assessment of patients with upper gastrointestinal hemorrhage. Clin Gastroenterol Hepatol 2012; 10:1130-5.
24. Hyett BH, Abougergi MS, Charpentier JP, et al. The AIMS65 score compared with the Glasgow-Blatchford score in predicting outcomes in upper GI bleeding. Gastrointest Endosc 2013; 77:551-7.
25. Cheng DW, Lu YW, Teller T, Sekhon HK, Wu BU. A modified Glasgow Blatchford Score improves risk stratification in upper gastrointestinal bleed: a prospective comparison of scoring systems. Aliment Pharmacol Ther 2012; 36:782-9.
26. Stanley AJ. Update on risk scoring systems for patients with upper gastrointestinal haemorrhage. World J Gastroenterol 2012; 18:2739-44.
27. Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000; 356:1318-21.
28. Ko BS, Kim YJ, Jung DH, et al. Early risk score for predicting hypotension in normotensive patients with non-variceal upper gastrointestinal bleedin. J Clin Med 2019; 8:37.
29. Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation. Lancet 2009; 373:42-7.
30. Bryant RV, Kuo P, Williamson K, et al. Performance of the Glasgow-Blatchford score in predicting clinical outcomes and intervention in hospitalized patients with upper GI bleeding. Gastrointest Endosc 2013; 78:576-83.
31. Jeffrey RB Jr, Federle MP. The collapsed inferior vena cava: CT evidence of hypovolemia. AJR Am J Roentgenol 1988; 150:431-2.
32. Liao YY, Lin HJ, Lu YH, Foo NP, Guo HR, Chen KT. Does CT evidence of a flat inferior vena cava indicate hypovolemia in blunt trauma patients with solid organ injuries? J Trauma 2011; 70:1358-61.
Fig. 1.Measurement of the maximal transverse diameter and the maximal anteroposterior diameter of the inferior vena cava (arrows). 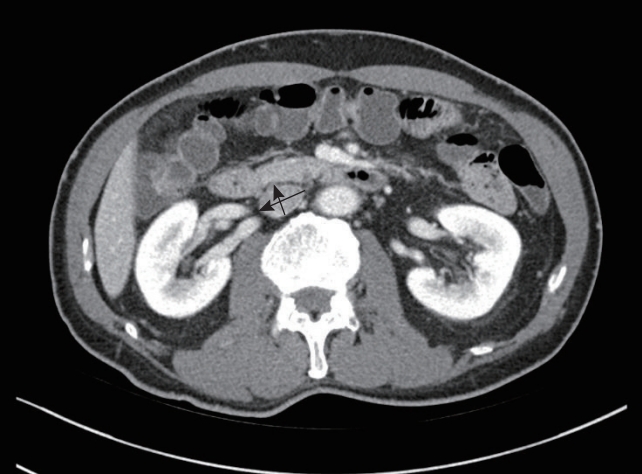
Fig. 2.Flow diagram showing the selection of the study population. CT, computed tomography; ED, emergency department. 
Fig. 3.Comparison of the inferior vena cava (IVC) diameter ratio with the Glasgow Blatchford score (GBS) and AIMS65 score for predicting in-hospital mortality. Area under the curve (AUC) of the IVC diameter ratio, 0.616 (95% CI, 0.498–0.735); AUC of the GBS, 0.472 (95% CI, 0.362–0.581); and AUC of the AIMS65 score, 0.496 (95% CI, 0.384–0.608). AIMS65, albumin <30 g/L (A), international normalized ratio >1.5 (I), altered mental state (M), systolic blood pressure ≤90 (S), and age >65 years (65). 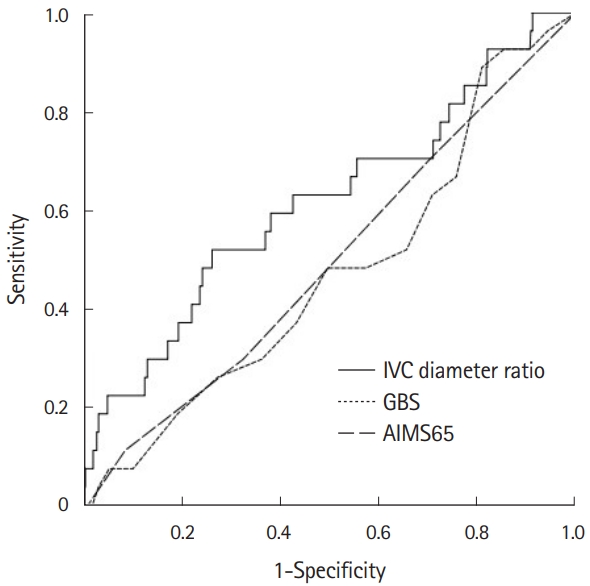
Table 1.Baseline characteristics of patients with gastrointestinal bleeding Values are presented as number (%) or median (interquartile range), unless otherwise indicated. SD, standard deviation; BUN, blood urea nitrogen; ICU, intensive care unit; AIMS65, albumin <30 g/L (A), international normalized ratio >1.5 (I), altered mental state (M), systolic blood pressure ≤90 (S), and age >65 years (65). Table 2.Multivariable logistic regression analysis of factors predicting in-hospital mortality The model was adjusted for age, sex, Glasgow Blatchford score, AIMS65 score, systolic blood pressure, heart rate, heart failure, hypertension, diabetes mellitus, and liver cirrhosis. CI, confidence interval; IVC, inferior vena cava; AIMS65, albumin <30 g/L (A), international normalized ratio >1.5 (I), altered mental state (M), systolic blood pressure ≤90 (S), and age >65 years (65). |
|
|||||||||||||||||||||||||||||||||||||||||