A multicenter, randomized, double-blind, placebo-controlled trial of amantadine to stimulate awakening in comatose patients resuscitated from cardiac arrest
Article information
Abstract
Objective
We hypothesized that the administration of amantadine would increase awakening of comatose patients resuscitated from cardiac arrest.
Methods
We performed a prospective, randomized, controlled pilot trial, randomizing subjects to amantadine 100 mg twice daily or placebo for up to 7 days. The study drug was administered between 72 and 120 hours after resuscitation and patients with absent N20 cortical responses, early cerebral edema, or ongoing malignant electroencephalography patterns were excluded. Our primary outcome was awakening, defined as following two-step commands, within 28 days of cardiac arrest. Secondary outcomes included length of stay, awakening, time to awakening, and neurologic outcome measured by Cerebral Performance Category at hospital discharge. We compared the proportion of subjects awakening and hospital survival using Fisher exact tests and time to awakening and hospital length of stay using Wilcoxon rank sum tests.
Results
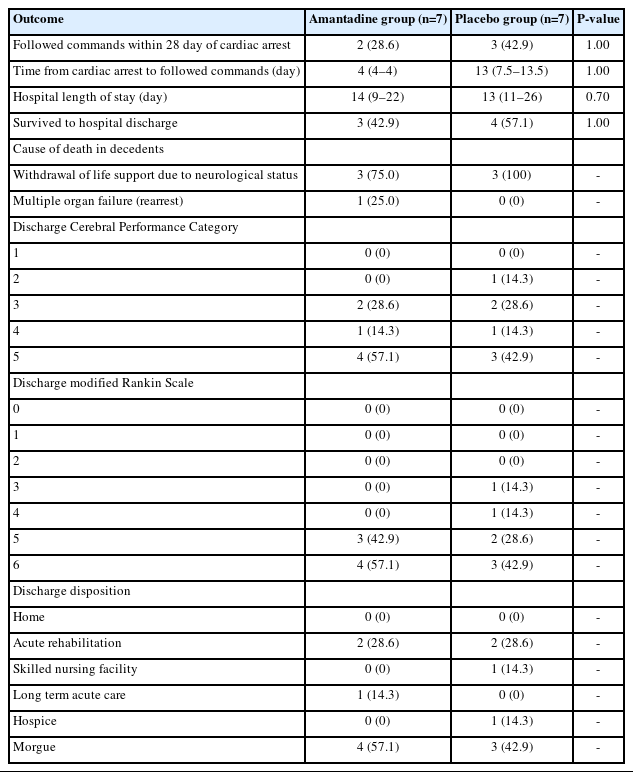
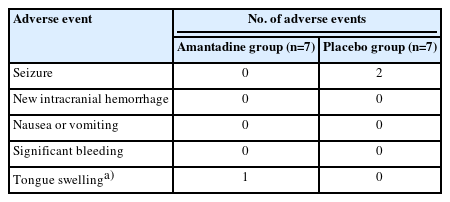
After 2 years, we stopped the study due to slow enrollment and lapse of funding. We enrolled 14 subjects (12% of goal enrollment), seven in the amantadine group and seven in the placebo group. The proportion of patients who awakened within 28 days after cardiac arrest did not differ between amantadine (n=2, 28.6%) and placebo groups (n=3, 42.9%; P>0.99). There were no differences in secondary outcomes. Study medication was stopped in three subjects (21.4%). Adverse events included a recurrence of seizures (n=2; 14.3%), both of which occurred in the placebo group.
Conclusion
We could not determine the effect of amantadine on awakening in comatose survivors of cardiac arrest due to small sample size.
INTRODUCTION
Cardiac arrest affects approximately 600,000 adults in the United States annually with a survival rate of just 9.1% [1]. For patients who remain comatose after return of spontaneous circulation, the most common cause for death is withdrawal of life-sustaining therapy for perceived poor neurological prognosis (WLST-N) [2–4]. Persistent coma is a strong risk factor for WLST-N which nearly universally results in death [5]. Interventions that promote awakening may improve outcomes.
Prior literature supports the potential safety and effectiveness of neurostimulants in patients with disorders of consciousness after severe acute brain injury. Amantadine is a commonly used neurostimulant with N-Methyl-D-aspartate (NMDA) and dopaminergic effects, both of which are implicated in functional and cognitive disability after traumatic brain injury [6]. In patients with subacute disorders of consciousness after brain trauma, treatment with amantadine resulted in faster functional recovery [7]. Amantadine may also improve consciousness in stroke patients with acute disorders of consciousness [8]. In our prior experience, amantadine was well-tolerated in patients resuscitated from cardiac arrest [9,10]. There have been no randomized trials of neurostimulant medications in patients with acute disorders of consciousness following resuscitation from cardiac arrest.
We performed a multicenter, randomized, double-blind, placebo-controlled pilot trial of amantadine in patients who remained comatose after successful resuscitation from cardiac arrest. We hypothesized amantadine would increase the rate of awakening while not increasing the rate of adverse events.
METHODS
Ethics statement
The study was approved by the Institutional Review Board at each participating institution (No. PRO15050414): University of Pittsburgh Medical Center (Pittsburgh, PA, USA), Beth Israel Deaconess Medical Center (Boston, MA, USA), and Maine Medical Center (Portland, ME, USA). Legally authorized representatives were approached as early as 48 hours after return of spontaneous circulation (ROSC). If the patient remained comatose 72 hours after ROSC the legally authorized representative was approached for informed consent. For subjects who later regained decision-making capacity, informed consent was obtained for ongoing participation in the trial.
Trial design
We conducted a multicenter, double-blind, randomized controlled trial at three high-volume cardiac arrest centers in the United States (ClinicalTrials.gov identifier: NCT02486211) between July 2016 and June 2017.
Participants
We included patients who were ≥18 years of age, received cardiopulmonary resuscitation for pulselessness regardless of location (in- or out-of-hospital), had a nontraumatic etiology of the cardiac arrest, and were comatose 72 hours after ROSC. Coma was defined as an inability to follow simple verbal commands. We excluded patients with prior written do-not-resuscitate orders, prisoners, pregnancy, lack of motor response to pain and absent N20 response on somatosensory evoked potential testing, cerebral edema on initial computed tomography scan of the brain (defined as a gray to white ratio of <1.20), malignant electroencephalography (EEG) pattern at the time of randomization, current use of other dopaminergic medications, and subjects whose prior wishes would not include supportive care for at least 1 week after enrollment. We considered generalized periodic discharges (regardless of background continuity), myoclonic status epilepticus, or nonconvulsive status epilepticus as interpreted by the site epileptologist to be malignant EEG patterns [11]. The original study design excluded subjects with a creatinine clearance of <50 mL/min. We removed this exclusion and added renal dosing of amantadine after the first year of enrollment.
Demographics and clinical characteristics
We recorded age, sex, race, location of arrest (in- or out-of-hospital), initial heart rhythm, illness severity as measured within 6 hours of cardiac arrest using the Pittsburgh Cardiac Arrest Category (PCAC) [12,13], Sequential Organ Failure Assessment (SOFA) cardiovascular and respiratory subscales [14], Full Outline of Unresponsiveness (FOUR) motor and brain subscales [15], length of hospital stay, survival to hospital discharge, discharge disposition, and neurologic (Cerebral Performance Category) and functional outcomes (modified Rankin Scale) assessed at hospital discharge.
Intervention
After informed consent, study drug was initiated between 72 and 120 hours following ROSC. We chose this time window to allow for the completion of neurological prognostic workups and for sedative drug clearance that could confound neurological examination. Subjects randomized to amantadine received 100 mg solution at 6:00 and 12:00 for 7 days. Subjects randomized to placebo received a dose of identical appearing liquid at the same time points.
Outcome measures
The primary outcome was the proportion of patients with awakening, defined as regaining the ability to follow simple two-step verbal commands, within 28 days after cardiac arrest. Study investigators performed daily neurological examinations during sedation pauses and recorded FOUR motor and brainstem components for the duration of hospitalization. Sedation was not protocolized in this study, but clinical practice at enrolling centers was to use short-acting sedation such as propofol or fentanyl infusions and avoid intermittent or continuous infusions of benzodiazepines. In cases where patients remained unconscious after hospital discharge, site study teams monitored patient status remotely to determine the occurrence of the primary outcome. As secondary outcomes, we recorded patient survival to hospital discharge, length of hospital stay, and in patients that awakened, time from start of study medication to awakening, in days. In decedents, we categorized death as WLST-N or rearrest due to refractory organ failure.
Sample size
We initially powered the trial to determine a relative difference in rate of awakening of 20% between groups (30% awakening in the control arm, 50% in awakening in the amantadine arm) with 80% power and a type 1 error rate of 5%. The goal sample size was 120 subjects.
Randomization and blinding
We randomized subjects 1:1 to amantadine or placebo in permuted blocks of two or four, stratified by institution, PCAC, and prior EEG findings. To maintain blinding of the treatment team, study drug was listed as “study drug” in the electronic medical record. Emergency unblinding could be requested by a treating physician but required discussion with the local and national principal investigator.
Adverse events
We defined suspected adverse drug events as development of seizures, intracranial hemorrhage, nausea and/or vomiting, significant bleeding, or other adverse events deemed possibly related to the study medication. Each reaction was adjudicated by the local site principal investigator.
Statistical analysis
We reported subject characteristics as mean and standard deviation, median and interquartile range (IQR), and number and percentage, as appropriate. We analyzed data using intention-to-treat principles. We plotted motor examination over the first 7 days after randomization as FOUR motor subscore for each subject. We compared the proportion of subjects that awakened in each trial arm during the study period using Fisher exact test. For secondary outcomes, we compared the proportion of subjects that survived to hospital discharge using Fisher exact test as well as time to awakening and hospital length of stay using Wilcoxon rank sum test. We considered a two-sided P-value of 0.05 as the threshold for statistical significance for all tests. We used R ver. 4.0.5 (R Foundation for Statistical Computing) for all analyses [16].
RESULTS
Study population
Between July 2016 and June 2017, we treated 385 cardiac arrest patients (Fig. 1). A total of 139 patients died before screening and 34 were screening failures. We screened 212 potential subjects and excluded cases noted in Fig. 1. We enrolled and randomized 14 subjects, seven to amantadine and seven to standard care, which accounted for 4% of all patients treated during the study period. There was no loss to follow-up. Study enrollment was stopped due to slow enrollment and lapse of funding. Baseline characteristics were similar between study groups (Table 1).
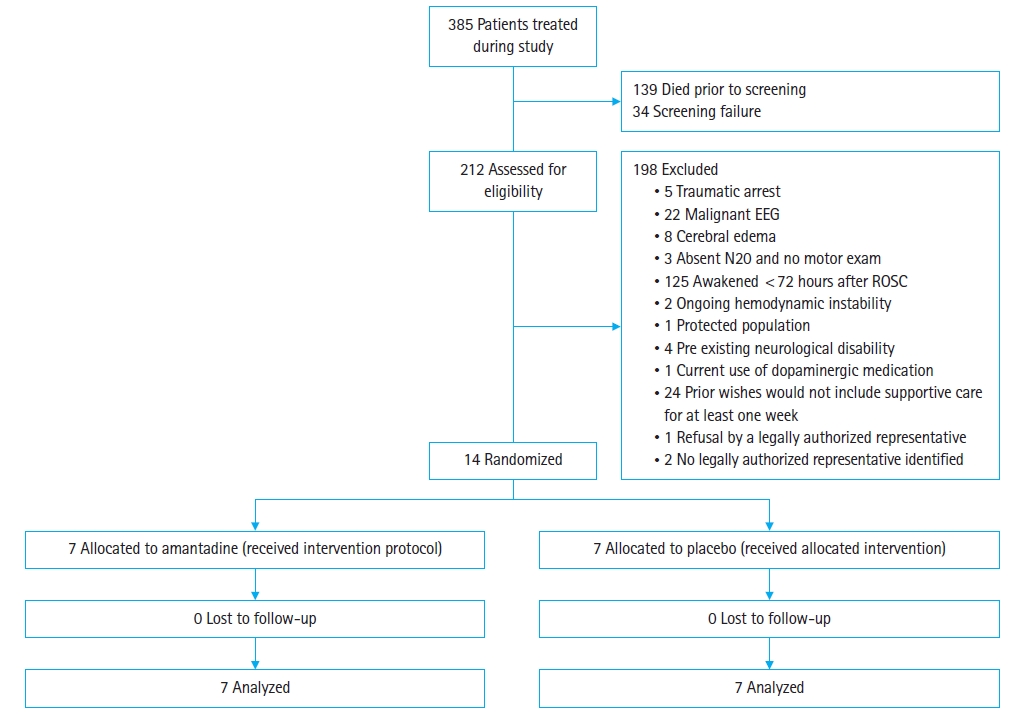
The CONSORT (Consolidated Standards of Reporting Trials) flowchart. EEG, electroencephalography; ROSC, return of spontaneous circulation.
Primary outcome
The proportion of patients who awakened within 28 days after cardiac arrest was not different between amantadine (n=2, 28.6%) and placebo groups (n=3, 42.9%; P>0.99).
Secondary outcomes
In patients who awakened within 28 days of arrest, time to awakening was 4 days (IQR, 4–4 days) in the amantadine group and 13 days (IQR, 7.5–13.5 days) in the placebo group (P=0.78) (Table 2). Hospital length of stay (amantadine group, 14 days [IQR, 9–22 days]; placebo group, 13 days [IQR, 11–26 days]; P=0.70) and survival (amantadine group, three patients [42.9%]; placebo group, four patients [57.1%]; P>0.99) was similar between groups. Trajectories of individual subject motor examinations are shown in Fig. 2. Cause of death in the seven subjects who died was hemodynamic instability (n=1) and WLST-N (n=6). Most survivors (n=4, 57.1%) were discharged to inpatient rehabilitation. Among decedents, death occurred a median of 9 days (IQR, 8.5–12.5 days) after ROSC.
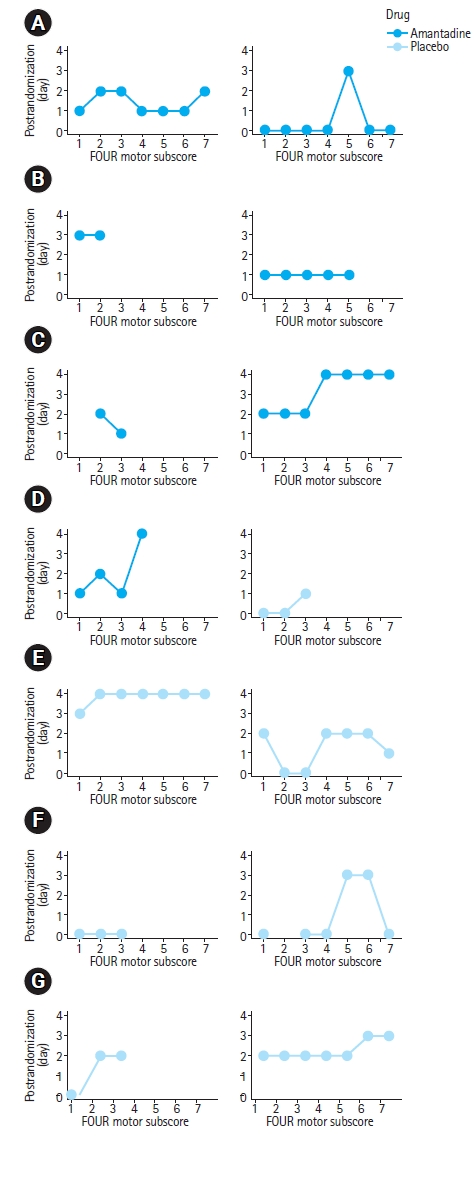
Subject level motor examinations by day of study. (A–N) Full Outline of Unresponsiveness (FOUR) score motor components are provided for all 14 enrolled subjects each day after randomization. Missing data is the result of neurological examination not being obtained or death prior to the completion of the treatment period.
Adverse events
Study medication was stopped in three subjects (Table 3). Adverse events included two recurrences of seizure, both of which occurred in the placebo group. One subject in the amantadine group had tongue swelling, which was determined to be unrelated to trial medication, though all enteral medications were held including amantadine.
DISCUSSION
The trial was stopped early due to slow enrollment and lapse of funding, leaving this study underpowered to detect our a priori effect size. As designed, only a small fraction of cardiac arrest patients (3.6%) were eligible for the study and a majority of potential subjects died or awoke before study screening. While amantadine did not increase awakening within 28 days in our planned statistical analysis, our small sample size limits our conclusion for any effect or lack thereof for amantadine in this population. No significant adverse drug events were attributed to amantadine. Awakening from coma after cardiac arrest can occur days after discontinuation of sedation [17], leaving patients at risk for WLST-N before recovery of consciousness. Hastening recovery of consciousness is a potential novel therapeutic target that may reduce excess mortality, though larger trials are needed to determine the effect of neurostimulants. Our experience highlights that such trials should undergo rigorous feasibility testing.
Our aim was to enroll cardiac arrest patients who did not have evidence of severe primary hypoxic-ischemic encephalopathy, remained comatose at 72 hours after ROSC, but also had preexisting wishes that aligned with longer trials of intensive care. The most common reasons for ineligibility were awakening from coma or death before eligibility for the study. While delayed emergence has been reported in approximately one-third of patients who ultimately awaken, this represents a small proportion of patients admitted to the intensive care unit after cardiac arrest [17,18]. Numerous risk factors for delayed emergence from postanoxic coma have been described that were not controlled for in our analysis including sedation regimen and longitudinal severity of extracranial organ failure [17–19]. Moreover, we excluded patients with prognostic markers of potentially severe brain injury, but it is unclear if these criteria excluded patients who might have benefited. Larger trials may allow for adequate sample size for statistical control of neurological illness severity and risk factors for delayed awakening as well as identify subgroups of treatment responders or nonresponders. Alternatively, determining specific phenotypes of patients most likely to benefit from neurostimulants could allow targeted enrollment.
We chose to trial amantadine given prior clinical observations, trial evidence in traumatic brain injury patients with subacute disorders of consciousness, and our prior work demonstrating acceptable side effect profiles [9,10]. Preclinical traumatic brain injury models and 123I-Ioflupane single-photon emission computed tomography imaging in patients with moderate-severe traumatic brain injury with cognitive impairments have observed reduced nigrostriatal dopamine levels [20,21]. Amantadine administration preserves dopamine levels in these brain regions, a mechanism by which it is thought to improve arousal and cognition after traumatic brain injury [22,23]. However, whole brain hypoxic-ischemic and reperfusion injury may cause different expressions of dopamine transmission. Nora et al. [24] observed augmented dopamine release in dorsal striatum and nucleus accumbens in a murine ventricular fibrillation model of cardiac arrest. This response was exacerbated in methylphenidate treated animals. Modafanil promotes awakening through multiple proposed mechanisms including increasing dopamine, norepinephrine, serotonin transmission, and reducing extracellular γ-aminobutyric acid [25], but to our knowledge, no preclinical mechanistic evidence is available in cardiac arrest. Additional preclinical evidence is needed to inform optimal neurostimulant medication selection.
Our primary endpoint was awakening, defined as the ability to follow simple verbal commands. We chose the outcome as it was patient-oriented and failure to awaken is associated with WLST-N. We note that this dichotomous outcome does not capture more subtle signs of improvement, including improved arousal, visual, or auditory function. Examinations such as the coma recovery score more thoroughly assess additional domains and capture subtle improvement, though they have primarily been used in the inpatient rehabilitation setting and are less well suited to the intensive care unit where we enrolled patients [26]. It is possible that evaluation of a prolonged trajectory of recovery (i.e., weeks to months) may yield different results.
Limitations
Our study has important limitations. We stopped enrollment before our prespecified sample size of 120 subjects. To enroll sample sizes large enough to detect small yet meaningful treatment effects, future trials will need to include many additional sites or use a continuous variable as the primary outcome such as time to awakening. To allow for completing neurological prognostication workups and excluding patients with highly malignant features, we started study drug between 72 and 120 hours after ROSC and continued for 7 days. Optimal drug, timing, and duration of neurostimulant medication is unknown. We enrolled patients in three cardiac arrest centers in the United States where most enrolled decedents died due to WLST-N. In addition to future trials requiring multiple centers to enroll adequate sample sizes, we suggest including sites from nations with infrequent withdrawal of life support to limit the potential for bias [27].
Conclusions
Patients who remain comatose after resuscitation from cardiac arrest tolerate amantadine well. In this setting, we were unable to recruit enough participants to make meaningful comparisons between amantadine and placebo in our planned statistical analysis.
Notes
Author contributions
Conceptualization: JCR; Data curation: PJC, JCR; Formal analysis: PJC, JCR; Funding acquisition: JCR; Investigation: all authors; Project administration: CWC; Resources: CWC; Supervision: JCR; Validation: PJC, JCR; Writing–original draft: PJC, JCR; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Conflicts of interest
Clifton W. Callaway is an Editorial Board member of Clinical and Experimental Emergency Medicine, but was not involved in the peer reviewer selection, evaluation, or decision process of this article. The authors have no other conflicts of interest to declare.
Funding
This study was supported by a grant funded by the American Heart Association (No. 15GRNT25680021).
Data availability
Data analyzed in this study are available from the corresponding author upon reasonable request.
References
Article information Continued
Notes
Capsule Summary
What is already known
Neurostimulant medications speed recovery in patients with subacute disorders of consciousness. While these medications have been used in patients with coma from cardiac arrest, no randomized controlled trials have tested their effectiveness in this population.
What is new in the current study
In this setting, we were unable to recruit enough participants to make meaningful comparisons between amantadine and placebo in our planned statistical analysis. Future trials should undergo rigorous feasibility testing and will require larger, multicenter cohorts for recruitment.