Association between the simultaneous decrease in the levels of soluble vascular cell adhesion molecule-1 and S100 protein and good neurological outcomes in cardiac arrest survivors
Article information
Abstract
Objective
This study aimed to determine whether simultaneous decreases in the serum levels of cell adhesion molecules (intracellular cell adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1], and E-selectin) and S100 proteins within the first 24 hours after the return of spontaneous circulation were associated with good neurological outcomes in cardiac arrest survivors.
Methods
This retrospective observational study was based on prospectively collected data from a single emergency intensive care unit (ICU). Twenty-nine out-of-hospital cardiac arrest survivors who were admitted to the ICU for post-resuscitation care were enrolled. Blood samples were collected at 0 and 24 hours after ICU admission. According to the 6-month cerebral performance category (CPC) scale, the patients were divided into good (CPC 1 and 2, n=12) and poor (CPC 3 to 5, n=17) outcome groups.
Results
No difference was observed between the two groups in terms of the serum levels of ICAM-1, VCAM-1, E-selectin, and S100 at 0 and 24 hours. A simultaneous decrease in the serum levels of VCAM-1 and S100 as well as E-selectin and S100 was associated with good neurological outcomes. When other variables were adjusted, a simultaneous decrease in the serum levels of VCAM-1 and S100 was independently associated with good neurological outcomes (odds ratio, 9.285; 95% confidence interval, 1.073 to 80.318; P=0.043).
Conclusion
A simultaneous decrease in the serum levels of soluble VCAM-1 and S100 within the first 24 hours after the return of spontaneous circulation was associated with a good neurological outcome in out-of-hospital cardiac arrest survivors.
INTRODUCTION
Out-of-hospital cardiac arrest is often fatal, and it has become an increasing social problem, with 4 to 5 million cases per year worldwide [1]. The survival rate of patients with cardiac arrest in Korea is 3% to 4%, and only 1.1% of all patients have good neurological outcomes (cerebral performance category [CPC] 1 and 2) [2].
Most patients who are successfully resuscitated from cardiac arrest are subjected to post-cardiac arrest care. Post-cardiac arrest syndrome (PCAS) is primarily due to global ischemia-reperfusion (IR) injury after successful resuscitation from cardiac arrest. During post-cardiac arrest period, microcirculatory endothelial dysfunction leads to the development of brain injury and multiple organ failure [3].
The blood-brain barrier (BBB) is a diffusion barrier that blocks the entry of most compounds from the blood to the brain. The BBB consists of three components of the brain microvasculature: endothelial cells, astrocyte end feet, and pericytes [4]. When the BBB is damaged, possible neurotoxic materials from the circulating blood can enter into the central nervous system, leading to neuronal injury [5,6].
Several markers used in predicting BBB injury in cardiac arrest victims were investigated, and S100 protein is a known potent marker [7-10], which is a part of a large family of calcium-binding proteins [11,12]. Although this protein is found in the glia and Schwann cells, it is not brain specific. It is found in other various cell types, such as adipocytes, melanocytes, skeletal muscle cells, and myoblasts [13]. Moreover, the elevated serum levels of S100 protein have been observed in various diseases, such as type 1 diabetes mellitus, Alzheimer’s disease, ischemic stroke, and traumatic brain injury [14-17]. Therefore, despite the studies on the relationships between serum S100 protein levels and neurological outcomes in cardiac arrest patients [14,15], the role of S100 protein in BBB destruction, particularly in IR injury, in cardiac arrest survivors has not been fully elucidated.
Endothelial dysfunction increases vascular permeability and promotes endothelium–leukocyte interactions resulting in systemic inflammation, which involves the activation of cell adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), and E-selectin [11,18]. BBB destruction is closely related to endothelial dysfunction [19]. Previous studies have shown that endothelial injury and BBB destruction occur in individuals after cardiac arrest and are associated with neurological outcomes [1,11,20]. Another study has shown that the simultaneous increase in plasma markers involved in endothelial activation and neuronal injury is associated with persistent delirium in critically ill patients [12]. However, no study on the relationship between endothelial injury and neurological outcomes in cardiac arrest individuals has been conducted [11,18,21].
In summary, an elevated level of S100 protein is not brain specific. However, it can be brain specific in individuals with BBB injury. Elevated levels of soluble cell adhesion molecules (ICAM-1, VCAM-1, and E-selectin) can help identify the degree of endothelial dysfunction, which is associated with BBB injury. Therefore, the degree of BBB injury can be identified based on the level of S100 protein along with the levels of soluble cell adhesion molecules.
We hypothesized that BBB destruction would contribute to the ongoing brain injury and poor neurological outcomes of cardiac arrest survivors, and the severity of BBB destruction can be identified based on the serum levels of S100 protein and soluble cell adhesion molecules. Thus, this study aimed to investigate whether simultaneous changes in the serum levels of S100 protein and soluble cell adhesion molecules (ICAM-1, VCAM-1, and E-selectin) within the first 24 hours after the return of spontaneous circulation (ROSC) were associated with the neurological outcomes in cardiac arrest survivors.
METHODS
Study setting and design
This retrospective observational study was based on prospectively collected data and approved by the institutional review board of Seoul National University Hospital (1702-155-836). This study was conducted between March 2013 and December 2015 at the emergency medical center of Seoul National University Hospital, Seoul, Korea.
We collected data and blood samples from out-of-hospital cardiac arrest survivors who had been admitted at the intensive care unit (ICU) for post-resuscitation care. Patients who collapsed due to cardiac causes were included. A written informed consent was obtained from each patient’s next of kin, and subsequently, if the patient regained consciousness, another consent was obtained from the survivor.
Patients who met any of the following criteria were excluded: patients aged under 18 years and admitted due to non-cardiac causes, including trauma, intoxication, drowning, and hanging, and those with prior advanced directives for Do Not Resuscitate, no written informed consent, insufficient blood samples, and loss of follow up after 6 months.
Clinical management
Hemodynamic support was provided to patients based on an early goal-directed therapy immediately after admission. Two or more electroencephalogram and, if necessary, brain magnetic resonance imaging tests were performed to evaluate complications and predict prognosis. All patients without contraindications (hemorrhage, fatal arrhythmia, hemodynamic instability, and sepsis) were subjected to targeted temperature management (TTM) in which the core body temperature was maintained at 33˚C for 24 hours, followed by rewarming (0.25˚C/hr).
Upon ICU admission, demographic data were collected. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score was obtained based on the data collected within the first 24 hours of admission. Blood samples were collected at 0 and 24 hours after admission.
Enzyme-linked immunosorbent assay
The serum levels of ICAM-1, VCAM-1, E-selectin, and S100 protein were measured using the enzyme-linked immunosorbent assay (ELISA) in duplicate. The Human ICAM-1/CD54 Allele-specific Quantikine ELISA Kit (DCD540; R&D Systems, Minneapolis, MN, USA), Human sVCAM-1/CD106 Quantikine ELISA Kit (DVC00, R&D Systems), Human sE-Selectin/CD62E Quantikine ELISA Kit (DSLE00, R&D Systems), and Human S100 ELISA Kit (MBS2503148; MyBioSource Inc., San Diego, CA, USA) were used, respectively.
BBB recovery data
Parameters that can be used as a basis for BBB recovery were defined as follows: -ΔS100 protein at 24 hours if S100 protein at 24 to 0 hours was less than 0; -ΔICAM-1 at 24 hours if ICAM-1 at 24 to 0 hours was less than 0; -ΔVCAM-1 at 24 hours if VCAM-1 at 24 to 0 hours was less than 0; and -ΔE-selectin at 24 hours if E-selectin at 24 to 0 hours was less than 0.
Outcome measures
According to the 6-month CPC scale, patients were divided into good (CPC scale 1 and 2) and poor (CPC scale 3 to 5) outcome groups.
Statistical analysis
Based on the study of the ICU patients who are still admitted, a sample size of at least 11 patients per group (a total of 22 persons or more) is required with 80% power to show a difference in the target protein expression (E-selectin serum concentration 30 vs. 40) between the groups at a 5% significance level.
Data were presented as median (ranges) or number (percentage) and compared between the groups via the Mann-Whitney U-test or Fisher exact test as appropriate. P-values<0.05 were considered statistically significant.
Multivariable analyses were performed to investigate the parameters independently associated with the primary outcome via stepwise logistic regression analyses (with an entry level of 0.05 and a retension level of 0.05) using variables with statistically significant differences between the good and poor neurological outcome groups. All statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Demographic and clinical characteristics
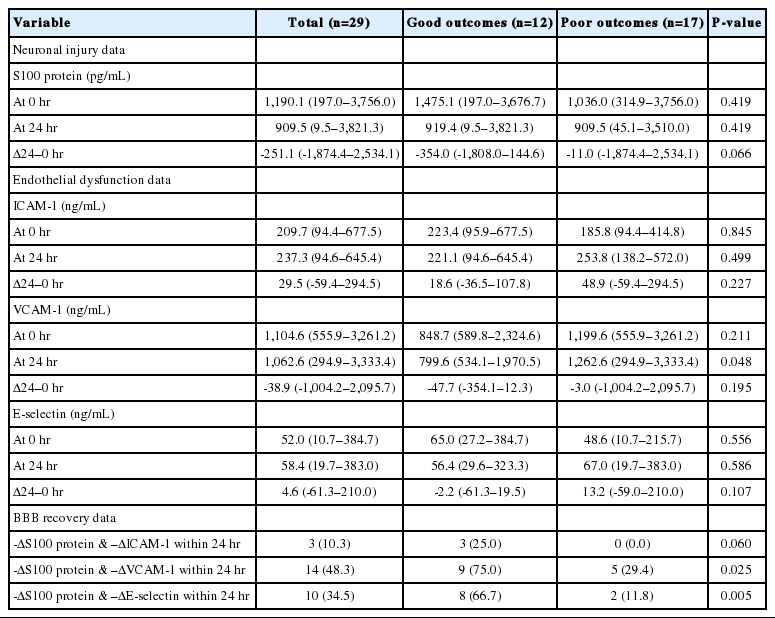
During the study period, 92 out-of-hospital cardiac arrest survivors were admitted to the ICU for post-resuscitation care. Of these patients, 29 were enrolled and 63 were excluded (Fig. 1). Baseline demographic and clinical characteristics are shown in Table 1. The median age of the participants was 67 years (range, 22 to 86 years). Nineteen (65.5%) patients were male, and 12 (41%) had good neurological outcomes. No significant differences were observed between the patients with good and poor neurological outcomes in terms of demographic and clinical characteristics, except for the length of time from cardiac arrest to ROSC and APACHE II score within the first 24 hours. Patients were managed according to general guidelines, including prespecified TTM protocols. No differences were observed between the good and poor neurological outcome groups in terms of the elapsed time it took to reach the target temperature, duration of rewarming, and core temperature rates during initial TTM and rewarming (Table 1 and Fig. 2).
Association between ICAM-1, VCAM-1, E-selectin, and S100 and neurological outcomes
The serum levels of ICAM-1, VCAM-1, E-selectin, and S100 at 0 and 24 hours were not different between the good and poor outcome groups, except that the serum level of VCAM-1 at 24 hours was higher in the poor outcome group than in the good outcome group. No differences were observed between the groups in terms of the changes in the serum levels of ICAM-1, VCAM-1, E-selectin, and S100 within 24 hours (Δ24–0 hr). However, when measuring the simultaneous changes in the serum levels of the above-mentioned molecules, more patients with good neurological outcomes had simultaneous decreases in the serum levels of both VCAM-1 and S100 as well as E-selectin and S100 (Table 2 and Fig. 3).

Scatter plots for the levels of (A) intracellular cell adhesion molecule-1 (ICAM-1) and S100 protein, (B) vascular cell adhesion molecule-1 (VCAM-1) and S100 protein, and (C) E-selectin and S100 protein. The red and blue dots represent the good and poor neurological outcome groups, respectively. CPC, cerebral performance category.
Based on the univariate analysis, simultaneous decreases in the serum levels of VCAM-1 and S100 (odds ratio, 7.200; 95% CI, 1.353 to 38.326; P=0.021) as well as E-selectin and S100 (odds ratio, 15.000; 95% CI, 2.239 to 100.483; P=0.005) were associated with good neurological outcomes. When adjusted for age, interval from arrest to ROSC, and the APACHE II score, only simultaneous decreases in VCAM-1 and S100 were independently associated with good neurological outcomes (odds ratio, 9.285; 95% CI, 1.073 to 80.318; P=0.043) (Table 3).
DISCUSSION
In the present study, we found that simultaneous decreases in the serum levels of VCAM-1 and S100 within the first 24 hours after the ROSC were associated with good neurological outcomes in cardiac arrest survivors. Based on this result alone, the mechanism is not fully elucidated. However, our results are consistent with the hypothesis that recovery from BBB destruction, which was identified using the simultaneous decreases in the protein markers for endothelial dysfunction and neuronal injury, was associated with good neurological outcomes in cardiac arrest survivors.
PCAS in patients who survive after cardiac arrest leads to vascular endothelial activation, which increases vascular permeability and promotes endothelium-white blood cell interaction and endothelium-mediated inflammation [18]. Several studies have shown that impaired endothelial reactivity is significantly associated with acute brain dysfunction in severe diseases. These data support the assumption that microcirculatory blood flow alteration due to endothelial dysfunction leads to serious organ dysfunction, including brain dysfunction in severe disease conditions [16,22,23]. In addition to the altered blood flow in the brain, endothelial dysfunction can contribute to increased BBB permeability and nerve damage because the BBB is composed of an endothelial layer with tight junctions and an astrocyte foot processes.
We collected blood samples at two time points (at 0 and 24 hours) according to previous studies. In one study about endothelial activation and injury after out-of-hospital cardiac arrest, out-of-hospital cardiac arrest survivors who were comatose showed altered levels of biomarkers for endothelial activation and damage within the first 72 hours, and significant changes in the levels of biomarkers were observed within 24 hours [11]. In another study, the serum levels of S100 protein in cardiac arrest survivors reached peak levels during the first hour, then decreased to the nadir before 24 hours [24]. However, no differences were observed between the good and poor outcome groups in terms of the levels of protein markers for either endothelial activation or neuronal damage at 0 and 24 hours after resuscitation. Compared with previous studies, the present study suggests that the levels of these markers measured at specific time points (0 and 24 hours) do not clearly reflect neurological outcomes. In addition, no significant association was observed between the decreased levels of any single marker within the first 24 hours after the ROSC and neurological outcomes. These results suggest that simultaneous changes in the levels of the markers for endothelial activation and neuronal injury may be important in predicting neurological outcomes.
Of the previously studied markers, S100 is the most relevant marker for BBB damage. In addition, the higher the serum concentration of S100 is, which is a significant marker of BBB damage, the more prolonged the patient’s delirium is. No difference was observed between the good and poor prognostic groups in terms of the S100 levels at 0 and 24 hours after resuscitation, which is similar to the results of other studies on the levels of endothelium markers. However, simultaneous decreases in the serum levels of VCAM-1 and S100 were associated with good neurological outcomes, which supports our hypothesis.
Furthermore, recent studies have shown that TTM can attenuate BBB destruction in various conditions such as global IR injury, stroke, and traumatic brain injury [25-27]. In the present study, all patients were treated with TTM according to prespecified protocols. Therefore, whether TTM attenuates BBB destruction or promotes recovery from BBB in cardiac arrest survivors could not be determined. Further research that focuses on the association between TTM and BBB destruction should be performed.
Cerebral autoregulation is a mechanism that helps maintain constant cerebral perfusion pressure when the mean arterial pressure changes [28]. Ameloot et al. [29] have conducted a study on impaired cerebral autoregulation in cardiac arrest victims, particularly in those with poor neurological outcomes, and have suggested that different hemodynamic targets for different individuals must be defined. Another study on the association between BBB disturbance and alterations in cerebral perfusion was also conducted [30]. Future studies must be conducted to show whether optimal hemodynamic targets change in cardiac arrest survivors under post-resuscitation care, which is according to the degree of BBB destruction.
The present study has limitations, mostly due to the nature of the study design itself. First, this is an observational study conducted at a single center, and it is difficult to generalize the results to a larger population. Therefore, larger scale multicenter study would be necessary. Second, a pathologic analysis was not performed because this was a human-based study. For this reason, we could not confirm whether the decreased levels of endothelial markers and S100 proteins may help in improving endothelial dysfunction and BBB injury. Well-designed in vivo or clinical studies should be conducted to support this hypothesis. Third, although not statistically significant, the proportion of patients with simultaneous decreases in the levels of both markers for endothelial dysfunction (ICAM-1, VCAM-1, and E-selectin) and neuronal injury (S100) were consistently higher in patients with good neurological outcomes than in those with poor neurological outcomes. Large-scale clinical studies with quantitative measurements of the levels of these biomarkers should be conducted to confirm our results that concomitant decreases in the markers for endothelial dysfunction and neuronal injury are associated with recovery from BBB destruction and good neurological outcomes in cardiac arrest survivors.
In conclusion, simultaneous decrease in VCAM-1 and S100 within the first 24 hours after the ROSC was independently associated with good neurological outcomes in cardiac arrest survivors who underwent TTM. Based on our data, we suggest that recovery from BBB destruction may be considered as a novel therapeutic target that improves neurological outcomes in cardiac arrest victims.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Blood-brain barrier (BBB) injuries occur in individuals with post-cardiac arrest syndrome, and endothelial dysfunction is significantly related to BBB injuries. S100 protein is a potent marker reflecting BBB injuries. However, its role is limited because the serum levels of S100 protein increases in individuals with various diseases other than cardiac arrest.
What is new in the current study
A simultaneous decrease in the serum levels of protein markers in individuals with endothelial dysfunction (vascular cell adhesion molecule-1) and neuronal injury (S100 protein), which reflects the recovery from BBB destruction, is associated with good neurological outcomes in out-of-hospital cardiac arrest victims.