New prehospital scoring system for traumatic brain injury to predict mortality and severe disability using motor Glasgow Coma Scale, hypotension, and hypoxia: a nationwide observational study
Article information
Abstract
Objective
Assessing the severity of injury and predicting outcomes are essential in traumatic brain injury (TBI). However, the respiratory rate and Glasgow Coma Scale (GCS) of the Revised Trauma Score (RTS) are difficult to use in the prehospital setting. This investigation aimed to develop a new prehospital trauma score for TBI (NTS-TBI) to predict mortality and disability.
Methods
We used a nationwide trauma database on severe trauma cases transported by fire departments across Korea in 2013 and 2015. NTS-TBI model 1 used systolic blood pressure <90 mmHg, peripheral capillary oxygen saturation <90% measured via pulse oximeter, and motor component of GCS. Model 2 comprised variables of model 1 and age >65 years. We assessed discriminative power via area under the curve (AUC) value for in-hospital mortality and disability defined according to the Glasgow Outcome Scale with scores of 2 or 3. We then compared AUC values of NTS-TBI with those of RTS.
Results
In total, 3,642 patients were enrolled. AUC values of NTS-TBI models 1 and 2 for mortality were 0.833 (95% confidence interval [CI], 0.815 to 0.852) and 0.852 (95% CI, 0.835 to 0.869), respectively, while AUC values for disability were 0.772 (95% CI, 0.749 to 0.796) and 0.784 (95% CI, 0.761 to 0.807), respectively. AUC values of NTS-TBI model 2 for mortality and disability were higher than those of RTS (0.819 and 0.761, respectively) (P<0.01).
Conclusion
Our NTS-TBI model using systolic blood pressure, motor component of GCS, oxygen saturation, and age was feasible for prehospital care and showed outstanding discriminative power for mortality.
INTRODUCTION
Traumatic brain injury (TBI) is among the major causes of mortality and disability worldwide [1]. In the United States, approximately 1.7 million patients with TBI were taken to the emergency department annually, and 52,000 patients died [2]. Moreover, 5.3 million patients have TBI-associated disability, which results in a socioeconomic burden [3-9].
Assessing injury severity and swift transport of the patient to the designated trauma center are essential to reduce the mortality in patients with severe trauma [10]. The mortality rate increased by two-fold when a patient with moderate to severe TBI was not transported to the designated trauma center according to prehospital field triage [11]. Therefore, measuring the injury severity and predicting the clinical outcome of TBI patients are critical to reduce secondary insult and improve outcome.
There are scoring systems to assess injury severity and predict outcome for TBI patients [12,13], but most include variables that are not commonly available in the routine prehospital setting, such as CT findings or laboratory results [12,14,15]. Recent studies reported that prehospital hypoxia and hypotension in patients with TBI increased mortality [16-19]; thus, systolic blood pressure (SBP) is a feasible indicator of mortality during the prehospital phase, and oxygen saturation measured via pulse oximeter is more objective and feasible than the respiratory rate (RR) included in the Revised Trauma Score (RTS) [20-24]. The Glasgow Coma Scale (GCS) is a practical method for assessing neurologic function, and the predictive power of the motor component of the GCS (motor GCS) is equivalent to that of the total GCS [25,26], which is used as a physiologic indicator in field triage [27,28].
This study aimed to develop a new trauma score optimized for prehospital care patients with TBI (NTS-TBI) to predict mortality and disability using motor GCS, hypotension, and hypoxia instead of total GCS, SBP, and RR of the RTS.
METHODS
Study design and setting
This was a retrospective, cross-sectional study that used an emergency medical services (EMS)-based severe trauma database operated by the Korea Center for Disease Control (KCDC) across South Korea. This study was approved by the study site’s institutional review board and the KCDC (H-1206-024-412). Informed consent was waived due to the retrospective nature of the study.
In South Korea, the EMS system is a nationwide public and single-tiered model operated by the fire department. Emergency departments are categorized into three levels: level 1, regional emergency medical center; level 2, local emergency medical center; and level 3, institutions not designated as regional or local emergency center across Korea [29]. Each province has designated regional trauma centers that are similar to level 1 trauma centers in the United States. Prehospital trauma care is provided by intermediate emergency medical technicians (EMT). EMTs used the field triage scheme to assess injury severity and transport patients to the designated facility. The field triage scheme used in Korea is similar to that developed by the Centers for Disease Control and Prevention in the United States [28].
Data source
The EMS-based severe trauma database (EMS-ST-DB) is a data source operated by the KCDC. Every case transported by the fire department categorized as a severe trauma case according to the field triage scheme is registered in the EMS-ST-DB.
The database collects prehospital information from the EMS run-sheets and in-depth severe trauma registry written by the EMTs of the fire department. Experienced medical record reviewers trained by the KCDC then visit the hospital receiving the severe trauma cases and collect in-hospital information and input final outcomes in the EMS-ST-DB. In 2013, the EMS-ST-DB collected information on cases from 10 provinces across Korea and expanded to all 17 provinces across Korea thereafter.
For quality improvement, a project quality management committee in the KCDC holds monthly meetings. This committee consisted of emergency physicians, epidemiologists, statistical experts, fire department representatives, and medical record reviewers. The quality management committee established the standard operational protocols for extracting data from the medical records and provided consultation and feedback to the medical record reviewers.
Study population
Among those enrolled in the EMS-ST-DB in 2013 and 2015, patients older than 18 years with TBI were included in the study. TBI was defined as a head injury according to the Abbreviated Injury Scale. The Abbreviated Injury Scale is a scoring system to measure the severity of injury for six body parts independently and is used for calculating Injury Severity Score [30]. We excluded patients who had prehospital traumatic arrest or those with non-traumatic injury. The exclusion criteria were as follows: (1) severe disability before the accident; (2) unknown or missing information on the variables in the NTS-TBI model, including SBP, RR, motor GCS, and age; and (3) a Glasgow Outcome Scale (GOS) of 2 or 3 before the injury. Briefly, the GOS is a global scale used to assess functional outcome; the score ranges from 1 to 5, with 1 indicating dead; 2, vegetative state; 3, severely disabled, dependent for daily support due to mental or physical disability, or both; 4, moderately disabled, able to work in a sheltered environment, and travel by public transportation; and 5, good recovery, resumption of normal life.
Model development and validation
We developed two NTS-TBI models using data from the EMS-ST-DB collected from 10 provinces in 2013. The first model of the NTS-TBI (NTS-TBI model 1) comprised motor GCS score, presence of shock defined as SBP <90 mmHg, and presence of hypoxia defined as oxygen saturation below 90% measured on pulse oximeter on initial assessment at the scene [17,31]. The second version of the NTS-TBI (NTS-TBI model 2) included all variables in model 1 and age older than 65 years. Old age was used in the second model as the elderly have been shown to have poor outcomes in previous research [13]. The cut-off for old age was set to ≥65 years based on the World Health Organization definition of the elderly [32].
We internally validated the predictive performance of NTS-TBI models 1 and 2 using the original data set used for model development. External validation was conducted using the EMS-ST-DB data collected from all 17 provinces in 2015.
Outcomes
The primary outcome was the predictive power of the NTS-TBI for in-hospital mortality, while the secondary outcome was the discriminative power of the model to predict severe disability at hospital discharge. Severe disability was defined as GOS score of 2 or 3 at hospital discharge. Cases with GOS score of 1 were excluded in the analysis of severe disability.
Comparison of the performance of the NTS-TBI and RTS
We compared the discriminative power for mortality and disability between the RTS and NTS-TBI models using the following equation: RTS=(0.9368×GCS score category)+(0.7326×SBP)+(0.2908 ×RR) [27]. The variables of RTS included in the equation were those measured by EMTs upon first contact with the patient at the field.
Statistical analysis
We performed descriptive analysis for demographic characteristics. Quantitative variables were reported as mean with standard deviation or median with interquartile range, and categorical variables were reported as number and percentage. We conducted the chi-square test of Fisher exact test for categorical variables and the t-test for continuous variables. To assess the predictive power of each model, we measured the area under the receiver operating characteristic curve and 95% confidence interval of each model. To compare the performance of the NTS-TBI and RTS, we compared the AUC value of each model and measured the significance of difference. Significance was set at P-values of 0.01, and all statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
There were 13,508 cases enrolled in the EMS-ST-DB during the study period; of these, 3,642 cases were included for model development and internal validation according to the inclusion criteria (Fig. 1). A total of 426 (11.7%) of the 3,642 cases died, and 452 cases had severe disability (Table 1). Mortality was higher in urban areas, during daytime, and in level 1 emergency departments. Mortality was also higher in the shock group than that in the non-shock group (28.2% vs. 10.5%, P<0.01) (Table 2) and in the hypoxia group than that in the non-hypoxia group (36.0% vs. 9.3%, P<0.01). The incidence of severe disability was also higher in the shock and hypoxia groups.
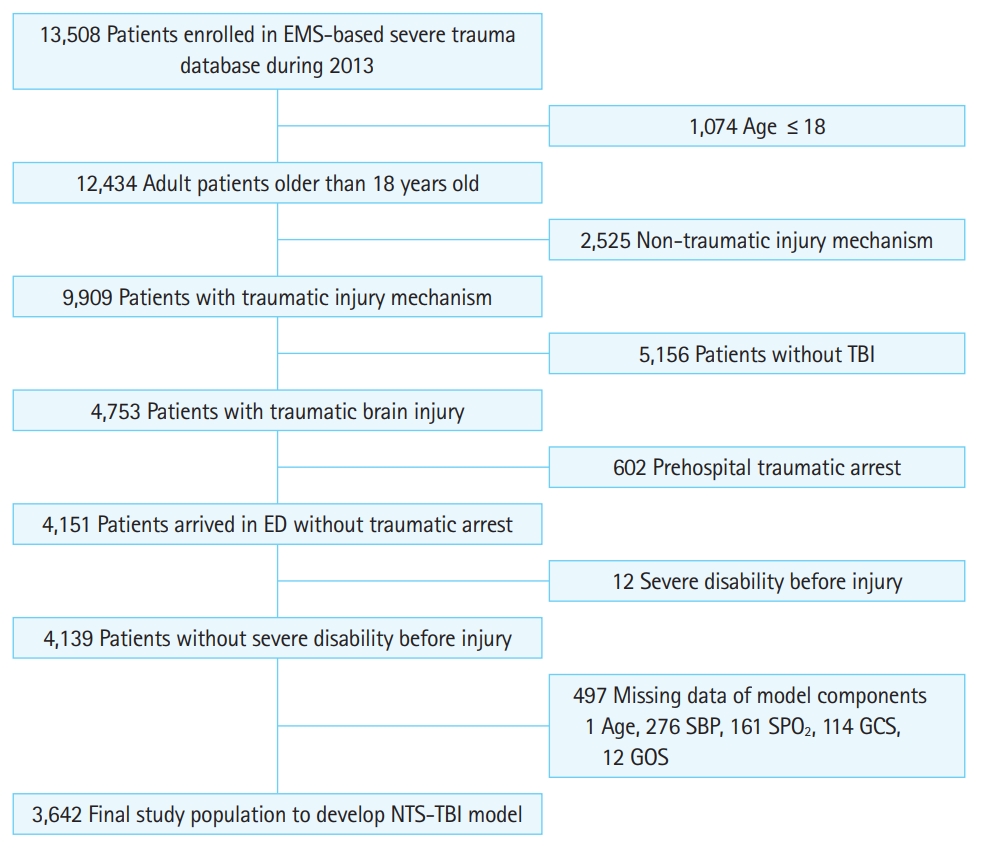
Inclusion criteria. EMS, emergency medical service; TBI, traumatic brain injury; ED, emergency department; SBP, systolic blood pressure; SPO2, oxygen saturation; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; NTS-TBI, new prehospital trauma score for traumatic brain injury.
We assessed the discriminative power of NTS-TBI models 1 and 2 by using the original data set (Table 3). The AUC value of NTS-TBI models 1 and 2 for in-hospital mortality was 0.833 (95% CI, 0.815 to 0.852) and 0.852 (95% CI, 0.835 to 0.869), respectively. The AUC of NTS-TBI models 1 and 2 for predicting severe disability was also higher than 0.75, indicating that the NTS-TBI model showed excellent performance in predicting outcome.
We also conducted an external validation by analyzing 4,497 of the 32,841 cases registered in the EMS-ST-DB from 17 provinces in 2015 (Table 4). The AUC value of NTS-TBI models 1 and 2 for in-hospital mortality was 0.779 (95% CI, 0.762 to 0.800) and 0.802 (95% CI, 0.786 to 0.828), respectively. The AUC for NTS-TBI models 1 and 2 to predict severe disability was also higher than 0.7, indicating that the NTS-TBI model showed good performance in predicting outcome.
We compared the predictive power of the NTS-TBI and RTS for mortality and severe disability (Table 5). The discriminative power of the NTS-TBI models 1 and 2 for mortality were 0.833 and 0.852, respectively, and they were significantly higher than that of the RTS (P<0.01). The AUC of the NTS-TBI models 1 and 2 for predicting severe disability was 0.772 and 0.784, respectively. NTS-TBI model 2 showed significantly better performance than that of RTS (AUC, 0.761) (P<0.01).
DISCUSSION
We developed a new scoring system to predict the clinical outcome for TBI patients and optimized it to be implemented in the prehospital care. We used motor GCS scores and oxygen saturation values obtained via pulse oximeter instead of total GCS score and RR used in the RTS. Motor GCS and oxygen saturation measured by pulse oximeter could be easily and objectively integrated into prehospital care. We developed two versions of the NTS-TBI model using the nationwide trauma registry operated by the KCDC. Both NTS-TBI models showed excellent performance as a predictor for mortality and good power for predicting severe disability in TBI patients. The discriminative power of NTS-TBI was significantly higher than that of RTS.
RTS has been used to predict outcomes for trauma patients, and the components of RTS are included in the first step of assessment in the field triage scheme [27,28,33]. However, some variables in the RTS are not easily available in the field. Thus, to improve ease of use and discriminative power, we developed an NTS-TBI. First, we replaced the GCS with the motor GCS, which has been shown to have a predictive power equivalent to that of total GCS in previous research. Moreover, the logistic model showed a more linear relationship with the outcome [25,26]. Second, hypoxia or hypotension during prehospital transport was included in the models as they have been shown to cause worse clinical outcomes such as mortality in recent studies [16-19]. Hypotension before emergency department arrival has a linear correlation with mortality [16]. Prehospital hypotension in TBI patients causes a 2.5-fold increase in mortality, and hypoxia results in a 3.0-fold increase. If combined, hypoxia and hypotension increased the risk of mortality by 6.1-fold [17]. Thus, assessing hypoxia and hypotension is essential during prehospital care to predict outcome. Third, RR has been used as a physiologic indicator to assess the severity of trauma. Given that it is difficult to measure the RR accurately in the prehospital stage [34,35], the pulse oximeter has been used as an objective tool to measure hypoxic status in diverse clinical settings [22-24], oxygen saturation reflects the patient’s respiratory function more objectively and efficiently [20,21]. The NTS-TBI included oxygen saturation instead of RR so that it can obtain more objective data and be used easily in the prehospital setting.
We reviewed diverse parameters for NTS-TBI. Age, GCS, RR, pupillary reactivity, hypotension, hypoxia, Injury Severity Score, injury mechanism, computed tomography images, and laboratory findings have been used to develop a trauma scoring system for TBI patients [12,13,15,36-39]. However, the Injury Severity Score, computed tomography images, and laboratory findings are not routinely available in the prehospital phase; thus, we developed a model that included variables that can be easily but objectively assessed during this phase.
This study has several limitations. First, the generalizability of the results is limited. Because we used data only from the EMS-ST-DB in Korea, and different trauma care systems vary among countries and could affect the performance of NTS-TBI. Second, there are inherent biases that may not have been eliminated due to the retrospective nature of the study. We retrospectively reviewed the medical records of each hospital and EMS run-sheet. Although we performed regular quality assessment, the quality of the raw medical record depends on each institution. Third, the variables in the NTS-TBI were limited to those in the EMS-ST-DB. For example, pupillary reactivity was not included and tested for model development.
In conclusion, the new trauma scoring system we developed to predict outcomes for TBI patients showed excellent performance to predict mortality. The NTS-TBI included variables that can be easily and objectively assessed in the pre-hospital setting, namely, motor GCS, hypoxia assessed using a pulse oximeter, shock, and age.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by the Korea Centers for Disease Control and Prevention.
References
Article information Continued
Notes
Capsule Summary
What is already known
Assessing injury severity and predicting outcomes are essential in traumatic brain injury (TBI). However, the respiratory rate and Glasgow Coma Scale of the Revised Trauma Score are difficult to use in the prehospital setting.
What is new in the current study
We developed a new prehospital trauma score for TBI model using systolic blood pressure, motor component of Glasgow Coma Scale, oxygen saturation, and age that was feasible for prehospital care. The new prehospital trauma score for TBI showed outstanding discriminative power for mortality with area under the curve values higher than 0.8.





