Usefulness of ischemia-modified albumin in the diagnosis of sepsis/septic shock in the emergency department
Article information
Abstract
Objective
No studies have evaluated the diagnostic value of ischemia-modified albumin (IMA) for the early detection of sepsis/septic shock in patients presenting to the emergency department (ED). We aimed to assess the usefulness of IMA in diagnosing sepsis/septic shock in the ED.
Methods
This retrospective, observational study analyzed IMA, lactate, high sensitivity C-reactive protein, and procalcitonin levels measured within 1 hour of ED arrival. Patients with suspected infection meeting at least two systemic inflammatory response syndrome criteria were included and classified into the infection, sepsis, and septic shock groups using Sepsis-3 definitions. Areas under the receiver operating characteristic curves (AUCs) with 95% confidence intervals (CIs) and multivariate logistic regression were used to determine diagnostic performance.
Results
This study included 300 adult patients. The AUC (95% CI) of IMA levels (cut-off ≥85.5 U/mL vs. ≥87.5 U/mL) was higher for the diagnosis of sepsis than for that of septic shock (0.729 [0.667–0.791] vs. 0.681 [0.613–0.824]) and was higher than the AUC of procalcitonin levels (cut-off ≥1.58 ng/mL, 0.678 [0.613–0.742]) for the diagnosis of sepsis. When IMA and lactate levels were combined, the AUCs were 0.815 (0.762–0.867) and 0.806 (0.754–0.858) for the diagnosis of sepsis and septic shock, respectively. IMA levels independently predicted sepsis (odds ratio, 1.05; 95% CI, 1.00–1.09; P=0.029) and septic shock (odds ratio, 1.07; 95% CI, 1.02–1.11; P=0.002).
Conclusion
Our findings indicate that IMA levels are a useful biomarker for diagnosing sepsis/ septic shock early, and their combination with lactate levels can enhance the predictive power for early diagnosis of sepsis/septic shock in the ED.
INTRODUCTION
Since the publication of the first edition of the International guidelines for management of sepsis and septic shock in 2004, many researchers have sought to promote the early diagnosis and appropriate treatment of sepsis and septic shock [1-4]. However, the mortality due to sepsis in patients remains between 20% and 30% worldwide [5]. The major causes of death in patients with sepsis are reduced intravascular volume, peripheral vasodilation, reduced myocardial function, and blood flow disturbances due to increased metabolism, which in turn results in an imbalance of oxygen distribution in the body; ultimately, this causes multiple organ dysfunction resulting from tissue hypoperfusion [6].
Therefore, aggressive fluid therapy and the appropriate use of antibiotics in the early stage of sepsis are the focus of sepsis and septic shock treatment, and the early detection and diagnosis of sepsis are the basis for undertaking systematic clinical measures [4,7,8]. Given that most patients with severe sepsis in the intensive care unit are triaged at the emergency department (ED), and only 53% of patients with sepsis are diagnosed in the ED, identifying a diagnostic tool that enables early diagnosis of sepsis in the ED is crucial [9,10]. There is currently no established biomarker for the early diagnosis of sepsis and septic shock; however, several biomarkers have been investigated as candidates [11-14]. Procalcitonin (PCT) is the most reliable independent biomarker for diagnosing sepsis. PCT and lactate are currently reported to be the most useful biomarkers for diagnosing severe sepsis and septic shock [14-16].
Ischemia-modified albumin (IMA) is albumin with structural modification at the N-terminal cobalt binding site due to free radicals released from ischemic tissues [17]. The usefulness of IMA was first reported in the early diagnosis of acute myocardial infarction [18-20], and its diagnostic effectiveness for various other ischemic diseases including stroke, pulmonary embolism, and ischemic vessel disease has also been reported [21-24]. It has been reported that IMA levels rise during sepsis and severe septic shock [25-27]. Khashana et al. [28] and Yerlikaya et al. [29] reported the usefulness of IMA in the early diagnosis of neonatal sepsis. However, there have been no reports on the diagnostic value of IMA for the early detection of sepsis and septic shock in patients presenting to the ED. This study aimed to investigate the usefulness of IMA in diagnosing sepsis and septic shock in ED patients according to the Sepsis-3 definitions [30] by comparing the diagnostic performances of PCT, lactate, high sensitivity C-reactive protein (hsCRP), and IMA levels in these cases.
METHODS
Study design
This retrospective, observational study included a consecutive patient cohort that had been admitted to the ED of a tertiary teaching hospital located in an urban area in Incheon, Korea. The Institutional Review Board of Gachon University Gil Medical Center approved this study (GCIRB2019-283). Due to the retrospective study design, the requirement for informed consent was waived.
Participant selection and data collection
Adult patients (aged ≥18 years old) who had visited the ED between July 1, 2018 and December 31, 2018 and showed at least two of the systemic inflammatory response syndrome criteria (fever >38.0°C or hypothermia <36°C; tachycardia >90 beats per minute; tachypnea >20 breaths per minute; and leukocytosis with white blood cell count >12,000/μL or leukopenia with white blood cell count <4,000/μL) were considered to have a potential infection. Patients who had undergone laboratory tests for IMA, PCT, lactate, and hsCRP levels in the ED within 1 hour of admission were included in this study. We retrospectively reviewed the electronic medical records of these patients. Patients with noninfectious conditions, such as trauma, thermal injury, sterile inflammation, pancreatitis, heart failure, drug and gas intoxication, gastrointestinal bleeding, seizure, cardiac arrhythmia, and shock due to non-infectious causes were excluded based on clinical and laboratory information. Patients who had been diagnosed with a disease known to elevate IMA levels [18-24], such as acute coronary syndrome, stroke, pulmonary embolism, peripheral vascular disease, and cardiac arrest, were also excluded, as were those who had undergone chemotherapy and those who required surgical intervention.
The following clinical and laboratory data were collected: age, sex, mean arterial pressure, length of hospital stay, 28-day hospital mortality, volumes of crystalloid infusion during ED stay, vasopressor use, and level of IMA, hsCRP, PCT, and lactate. The sequential organ failure assessment (SOFA) score and quick sepsis-related organ failure assessment (qSOFA) were calculated using the poorest physiologic results observed. Data of laboratory parameters measured within 1 hour of ED admission were collected.
Infection sources and microorganisms isolated from blood cultures
The source of infection was divided into six categories: respiratory infection (including pneumonia), urogenital infection, hepatobiliary infection, gastrointestinal infection, and other infections. When multiple infections were present, they were combined for calculations. For the identification of microbial pathogens, sampling and transportation of blood cultures were performed according to standard procedures.
Definition of sepsis and septic shock based on the Sepsis-3 guidelines
The Sepsis-3 guidelines redefined sepsis as life-threatening organ dysfunction due to a dysregulated host response to infection. Organ dysfunction can be identified as an acute change of ≥2 points in the total SOFA score subsequent to infection. Septic shock is defined as a state in which vasopressors are required to maintain the mean arterial pressure at ≥65 mmHg and serum lactate levels at >2 mmol/L (>18 mg/dL), despite the use of adequate fluid resuscitation [4].
Definition and application of the qSOFA
The qSOFA has three items: altered mentation, systolic blood pressure (≤100 mmHg), and a respiratory rate ≥22 bpm. A qSOFA score ≥2 has been reported to be a meaningful predictor of sepsis and poor outcomes [4,30,31].
Albumin cobalt binding test
IMA levels were measured using the albumin cobalt binding test (Medical System Biotechnology, Ningbo, China) on an ADVIA Chemistry XPT analyzer (Siemens Healthineers, Erlangen, Germany) according to the manufacturer’s specifications; this test has been previously validated [18].
Statistical analysis
Categorical variables were presented as the number of patients (%), and continuous variables were presented as the mean±standard deviation or median (interquartile range). For intergroup comparisons, categorical variables were analyzed using Chi-square tests and continuous variables were analyzed using independent t-tests or the Mann-Whitney U-test and Kruskal-Wallis test. All data were verified for normality using the Kolmogorov-Smirnov test.
To compare the diagnostic power for sepsis and septic shock based on the Sepsis-3 definitions and to determine the cut-off values, area under receiver operating characteristic (ROC) curve (AUC) analysis (with 95% confidence intervals [CIs]) was performed for PCT, lactate, hsCRP, and IMA levels, and for the qSOFA score. To determine the optimal cut-off value with maximum sensitivity and specificity, the Youden index (sensitivity+[specificity-1]) was calculated.
A multivariate logistic regression model with backward stepwise elimination was applied to identify independent predictors of sepsis and septic shock. Significant variables with P-values ≤0.10 in the univariate analysis were eligible for assessment in the multivariate logistic regression model. The variance inflation factor (VIF) was used as an indicator of autocorrelation or collinearity of independent variables included in the model. When the value of the VIF is >10, multicollinearity is considered to be high. Odds ratios (ORs) and their 95% CIs for variables selected using the logistic model were also calculated.
Statistical analysis was performed using IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA) software, and P-values <0.05 were considered statistically significant.
RESULTS
Clinical characteristics of the study population
In total, 557 patients were originally included in this study. Of these, 46 patients diagnosed with non-infectious disease, 207 patients diagnosed with a disease that could elevate IMA levels, and four patients with incomplete data were excluded. Finally, 300 patients were enrolled (Fig. 1). Of these, 148 (50.7%) patients were male; the median age was 75 (range, 66–81) years old; and 82, 134, and 84 patients had infection, sepsis, and septic shock, respectively. There were no significant sex distribution differences among the three groups (P=0.257). Patients with septic shock were the oldest (P<0.001). All patients with septic shock met the sepsis criteria according to the Sepsis-3 definitions. Moreover, the patients with septic shock had the highest levels of hsCRP, lactate, PCT, and IMA (P<0.001), and the highest SOFA and qSOFA scores (P<0.001). The length of hospital stay was significantly longer for patients with septic shock (13 days; range, 7–25.5) than for patients with infection or sepsis (0 days; range, 0–0 days and 10 days; range, 5–16 days, P<0.001), respectively (Table 1).
Flow chart of patient selection based on inclusion and exclusion criteria. ED, emergency department; SIRS, systemic inflammatory response syndrome; IMA, ischemia-modified albumin; PCT, procalcitonin; hsCRP, high sensitivity C-reactive protein; PCAS, post cardiac arrest syndrome; PTE, pulmonary thromboembolism.
Classification according to the source of infection and microbial identification
Table 2 shows the classification according to the source and frequency of infection. Respiratory infections, including pneumonia, were the most common source of infection (n=118, 39.3%), followed by urogenital infections (n=87, 29.0%), hepatobiliary infection (n=27, 9.0%), and infections of unknown cause or infections not classified into any of the above categories (n=56, 18.7%). Of the 300 patients, 104 (34.7%) were found to have positive blood culture results; 66 (22%) of these patients had gram-negative bacillus infections, 32 (10.67%) had gram-positive coccus infections, 2 (0.67%) had polymicrobial infections, and 3 (1%) had fungal infections (Candida albicans). Escherichia coli was the most common pathogen (42 patients, 14%), followed by Klebsiella pneumoniae, which was identified in 14 (4.67%) patients (Supplementary Table 1).
Predictability of sepsis for each biomarker
Of the 300 patients, 218 (72.7%) had sepsis, while 82 (27.3%) had an infection according to the Sepsis-3 definitions. The ROC curve for sepsis prediction is shown in Fig. 2. IMA levels had the highest AUC values at 0.729 (95% CI, 0.667–0.791; cut-off ≥85.5 U/mL). Sensitivity and specificity were 58.7% and 79.3%, respectively. The AUC values for lactate, PCT, and hsCRP levels were 0.722 (95% CI, 0.663–0.782; cut-off ≥2.35 mmol/L), 0.678 (95% CI, 0.613–0.742; cut-off ≥1.58 ng/mL), and 0.667 (95% CI, 0.601–0.733), respectively.

Comparison of the receiver operating characteristic curves of ischemia-modified albumin (IMA), procalcitonin (PCT), lactate, and high sensitivity C-reactive protein (hsCRP) levels for the prediction of (A) sepsis and (B) septic shock. AUC, area under receiver operating characteristic curve; CI, confidence interval.
Predictability of septic shock for each biomarker
According to the Sepsis-3 definitions, 84 of 300 (28.0%) patients were diagnosed with septic shock, and 216 (72.0%) patients were diagnosed with other infections. The ROC curve for septic shock prediction is shown in Fig. 2. Lactate levels had the highest AUC value at 0.751 (95% CI, 0.686–0.814) and cut-off value of >2.35 mmol/L. Sensitivity and specificity were 66.7% and 75.0%, respectively. The AUC value for PCT levels was 0.714 (95% CI, 0.648–0.779) with a cut-off value of >2.67 ng/mL. Sensitivity and specificity were 60.7% and 72.7%, respectively. The AUC for IMA levels was 0.681 (95% CI, 0.613–0.824) with a cut-off value of >87.5 U/mL. Sensitivity and specificity were 58.3% and 71.3%, respectively. The AUC for hsCRP levels was 0.615 (95% CI, 0.546–0.683).
Identification of independent predictors of sepsis and septic shock using multivariate logistic regression analysis
Using multivariate logistic regression analysis, lactate (OR, 1.85; 95% CI, 1.36–2.50; P<0.001), hsCRP (OR, 1.05; 95% CI, 1.02–1.10; P<0.004), IMA (OR, 1.05; 95% CI, 1.00–1.09; P<0.029), and PCT (OR, 1.05; 95% CI, 0.99–1.10; P<0.099) levels were found to be independent predictors of sepsis in patients with infection. Regarding septic shock, multivariate logistic regression analysis indicated that lactate (OR, 1.31; 95% CI, 1.13–1.53; P<0.001), IMA (OR, 1.07; 95% CI, 1.02–1.11; P<0.002), and PCT (OR, 1.01; 95% CI, 1.00–1.03; P<0.027) levels were independent predictors, but hsCRP levels were not (Table 3). There was no evidence of autocorrelation or collinearity among the variables as the VIF for each pair of variables was <10 (data not shown).
Comparison of the predictive power of IMA levels combined with other biomarker tests and the qSOFA score for sepsis and septic shock
The diagnostic AUC for sepsis was the highest when IMA levels were combined with lactate levels (0.815 [95% CI, 0.762–0.867]; sensitivity, 78.4%; specificity, 73.2%). The AUC was 0.765 (95% CI, 0.707–0.824) when IMA levels were combined with PCT levels and 0.713 (95% CI, 0.546–0.683) when they were combined with hsCRP levels. When IMA levels were combined with the qSOFA score, the AUC was 0.858 (95% CI, 0.817–0.9; sensitivity, 66.1%; specificity, 92.7%).
The diagnostic AUC for septic shock was the highest when IMA levels were combined with lactate levels (0.806 [95% CI, 0.754–0.858]; sensitivity, 81.0%; specificity, 65.3%). The AUC was 0.771 (95% CI, 0.712–0.830) when IMA levels were combined with PCT levels and 0.667 (95% CI, 0.602–0.732) when they were combined with hsCRP levels. When IMA levels and the qSOFA were combined, the AUC was 0.882 (95% CI, 0.842–0.923; sensitivity, 77.4%; specificity, 82.9%). The details are shown in Fig. 3.
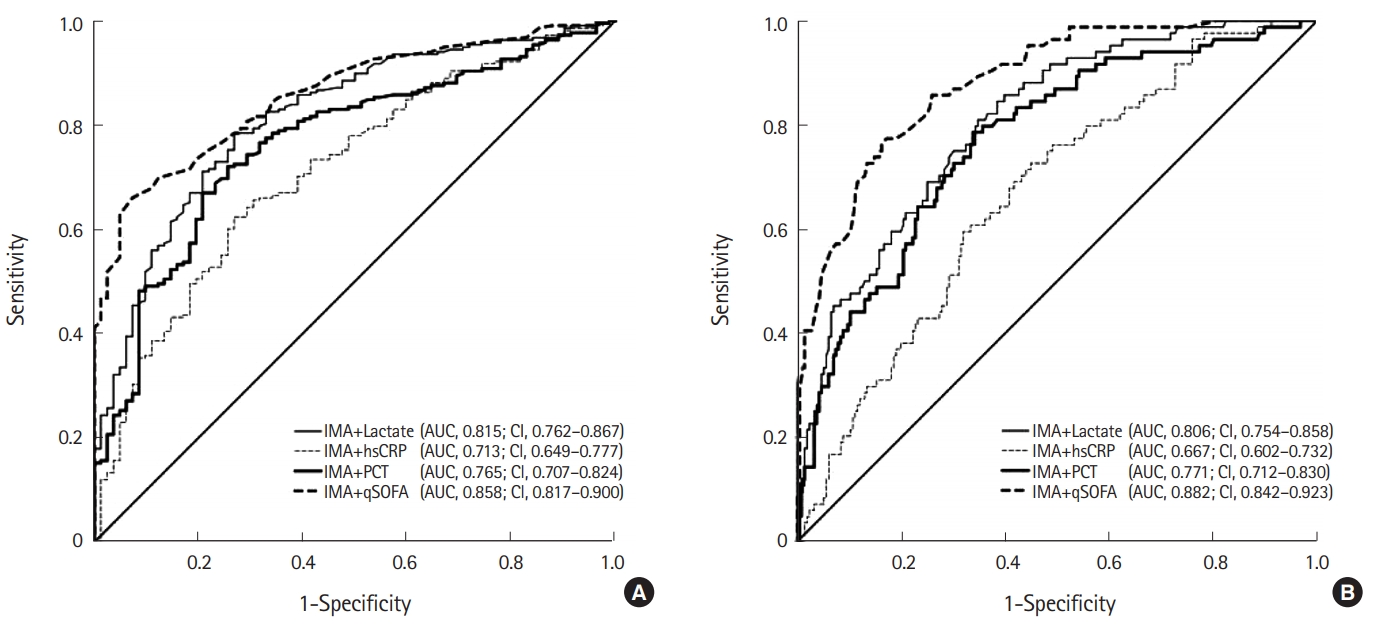
Comparison of receiver operating characteristic curves of procalcitonin (PCT), lactate, and high sensitivity C-reactive protein (hsCRP) levels, and quick sepsis-related organ failure assessment (qSOFA) score combined with ischemia-modified albumin (IMA) levels for (A) sepsis and (B) septic shock prediction. AUC, area under receiver operating characteristic curve; CI, confidence interval.
Value of the qSOFA for predicting sepsis and septic shock
For qSOFA scores ≥2, the AUC for the diagnosis of sepsis was 0.820 (95% CI, 0.772–0.867). Sensitivity and specificity were 44.5% and 99.8%, respectively. The AUC for the diagnosis of septic shock was 0.870 (95% CI, 0.828–0.912). Sensitivity and specificity were 75.0% and 83.8%, respectively.
DISCUSSION
Early diagnosis of sepsis in patients with infection in the ED is essential to start targeted therapy as quickly as possible. Although the Sepsis-3 definitions, revised according to the SOFA scoring system, provides guidelines to detect and diagnose sepsis, calculating the SOFA score is time-consuming, as multiple laboratory tests and data are needed. Such assessments are nearly impossible to perform in a crowded environment such as the ED, where many patients require urgent care. Hence, various biomarkers that require less time for assessment and diagnosis are utilized for early diagnosis, differential diagnosis, risk stratification, treatment monitoring, and prognostic evaluation. PCT, lactate, and hsCRP levels are several standard biomarkers used for this purpose.
Organ failure in sepsis and septic shock has been reported to result from ischemia-induced hypoxic damage and free radicalinduced tissue damage [32,33]. The same mechanism has been reported to elevate IMA levels [30]. Ultimately, we inferred that IMA levels would be elevated in patients with sepsis or septic shock.
To our knowledge, this study is the first to demonstrate the usefulness of serum IMA levels as a predictor of sepsis and septic shock in patients with infection presenting to the ED. First, we investigated whether IMA levels could distinguish patients with sepsis from those without sepsis. At a cut-off of 85.5 U/mL, IMA levels were a significant predictor of sepsis (sensitivity, 58.7%; specificity, 79.3%; P<0.001). Moreover, IMA levels had the highest AUC (0.729) among all the biomarkers; this value was comparable to that of lactate levels (0.722) and higher than that of PCT (0.678) levels. Using logistic regression analysis, lactate, hsCRP, and IMA levels were found to be independent predictors of sepsis.
Second, we determined the usefulness of IMA in differentiating septic shock from other infections. At a cut-off of 87.5 U/mL, IMA levels differentiated patients with septic shock from those without septic shock (sensitivity, 58.3%; specificity, 71.3%). The AUC of IMA levels was 0.681, the third highest after that of lactate (0.751) and PCT (0.714) levels. As reported in a previous study, IMA, PCT, and lactate levels are meaningful markers for the monitoring and diagnosis of sepsis and sepsis-induced organ dysfunction, oxidative stress, and lactic acidosis in patients who are critically ill [27]. Using logistic regression analysis, we identified that lactate, IMA, and PCT levels were independent predictors of septic shock in patients with infection, but hsCRP levels were not.
As sepsis progresses to septic shock, tissue hypoperfusion occurs, leading to worsening of lactic acidosis. As a result, the diagnostic value of lactate seems to rise in patients with severe sepsis and septic shock, consistent with previous reports showing that a high lactate concentration reflects critical tissue hypoxia and severity [14,34,35].
The AUC of IMA levels for septic shock was significant at 0.681; however, it was lower than that for sepsis. This finding was in contrast to our expectation that the predictive power of IMA would be enhanced as sepsis progresses to septic shock, as was the case for lactate. This may be because the elevation of lactate levels could have led to a lower IMA value, as reported in a previous study [36]; however, further studies are needed to clarify whether this is an issue with the measurement method or a result of the characteristics of IMA. Moreover, when a test was conducted by combining IMA and lactate levels for the diagnosis of sepsis and septic shock, the AUCs were 0.815 and 0.805, respectively (sensitivity, 78.4 [81%]; specificity, 73.2 [65.3%]). These results demonstrated that the predictive power, sensitivity, and specificity of IMA and lactate levels combined were all higher than those for IMA or lactate alone. Based on these results, additional studies are needed to determine the cut-off values and the protocol for clinical application.
Third, we examined the usefulness of the qSOFA score alone and the qSOFA score combined with IMA levels in the differential diagnosis of sepsis and septic shock. Since the introduction of the Sepsis-3 definitions, the qSOFA score has been used as a prognostic predictor and sepsis screening tool [4,31]. In particular, the qSOFA is quick, simple, and does not require additional blood work, which adds to its usefulness in the ED. Studies on the diagnosis of sepsis, septic shock, and organ dysfunction outside the intensive care unit, particularly in the ED, have been performed [37,38]. Previous studies have reported that the qSOFA score had high diagnostic specificity (>90%) but low sensitivity (10%–30%) [37,39]. Our findings were similar, in that a qSOFA score of ≥1 had diagnostic sensitivity and specificity of 44.5% and 99.8% for sepsis, respectively, while a qSOFA score ≥2 had a diagnostic sensitivity and specificity of 14.7% and 99.9%, respectively. With such a low sensitivity, the clinical use of the qSOFA as a diagnostic tool for sepsis has been limited. Efforts to enhance its predictive power by combining it with biomarkers such as lactate and PCT levels have met with some success [40,41].
In this study, we combined IMA levels and the qSOFA score to predict sepsis and septic shock and observed that the combined predictive power (AUCs of 0.858 and 0.882, respectively) was higher than that of the qSOFA score (AUCs of 0.820 and 0.870, respectively) and IMA levels (AUCs of 0.729 and 0.681, respectively) alone. These results suggest that adding IMA to qSOFA as a diagnostic tool for sepsis and septic shock could compensate for the low sensitivity of qSOFA alone.
This study had several limitations. This single-center study had a limited study period and a relatively small population of 300 patients and may have had a regional selection bias. In general, patients admitted to the ED were tested and treated according to the Surviving Sepsis Campaign guidelines [4]. As each patient was attended to by different medical staff, treatment could not be performed according to completely standardized guidelines and within a consistent timeframe. Further, our data may have low reliability due to the retrospective nature of the study, and selection bias may have occurred during the inclusion and exclusion process.
When selecting the study population, patients who met at least two of the systemic inflammatory response syndrome criteria and those who had been tested for PCT, IMA, lactate, and hsCRP levels were considered. Due to internal guidelines and national insurance policies, tests for biomarkers (with the exception of hsCRP) were performed for patients with suspected sepsis and for those who showed unstable vital signs and appeared to be severely ill. Additionally, because of the nature of the wide regional emergency medical center, there were numerous severely ill and transferred patients; therefore, the study population may have been biased towards severely ill patients. This may have resulted in a higher prevalence of sepsis and septic shock among patients in our study population than among the general patient population who presents to an ED. Therefore, a prospective, multicenter study with a large study population from multiple regions is needed to address these shortcomings. Moreover, subsequent studies should examine the clinical value of IMA in risk stratification, treatment monitoring, and prognostic evaluation for patients with sepsis and assess the efficient clinical application of this biomarker.
In conclusion, IMA levels alone had diagnostic value for sepsis and septic shock among patients in the ED, in accordance with the Sepsis-3 definitions. Further, their predictive power could be enhanced when combined with lactate levels or the qSOFA score. IMA levels can serve as an important biomarker for the early diagnosis of sepsis and septic shock.
Notes
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIAL
Supplementary Table is available from: https://doi.org/10.15441/ceem.19.075.
Types of microorganisms isolated from blood cultures
References
Article information Continued
Notes
Capsule Summary
What is already known
The utility of ischemia-modified albumin was first recognized for the early diagnosis of acute myocardial infarction. Its diagnostic effectiveness for various diseases, including stroke, pulmonary embolism, ischemic vessel disease, sepsis, and septic shock, has also been reported.
What is new in the current study
This study showed the usefulness of ischemia-modified albumin in early diagnosis of sepsis/septic shock in the emergency department based on Sepsis-3 definitions.



