Use of noninvasive volume assessment methods to predict acute blood loss in spontaneously breathing volunteers
Article information
Abstract
Objective
The use of noninvasive volume assessment methods to predict acute blood loss in spontaneously breathing patients remains unclear. We aimed to investigate changes in the pleth variability index (PVI), vena cava collapsibility index (VCCI), end-tidal carbon dioxide (EtCO2), pulse pressure (PP), and mean arterial pressure (MAP) in spontaneously breathing volunteers after acute loss of 450 mL blood and passive leg raise (PLR).
Methods
This prospective observational study enrolled healthy volunteers in the blood donation center of an academic hospital. We measured the PVI, EtCO2, VCCI, MAP, and PP before blood donation; at the 0th and 10th minute of blood donation; and after PLR. The primary outcome was the changes in PVI, EtCO2, VCCI, MAP, and PP.
Results
We enrolled thirty volunteers. There were significant differences among the four obtained measurements of the PVI, EtCO2, and MAP (P<0.001, P<0.001, P<0.001, respectively). Compared to the predonation values, post-hoc analysis revealed an increase in the PVI at the 0th min postdonation (mean difference [MD], 5.4±5.9; 95% confidence interval [CI], -7.6 to -3.1; P<0.001); a decrease in the EtCO2 and MAP at the 0th and 10th minute postdonation, respectively (MD, 2.4±4.6; 95% CI, 0.019 to 4.84; P=0.008 and MD, 6.4±6.4; 95% CI, 3 to 9.7; P<0.001, respectively). Compared with EtCO2 at the 10th minute, the value increased after PLR (MD, 1.8±3.2; 95% CI, 0.074 to 4.44; P=0.006).
Conclusion
The PVI and EtCO2 could detect early hemodynamic changes after acute blood loss. However, it remains unclear whether they can determine volume status in spontaneously breathing patients.
INTRODUCTION
Hypovolemia is among the most common causes of circulatory failure in critically ill patients [1]. Early hypotension detection and determining its underlying reason could improve the outcome in the emergency department (ED) [2]. Generally, fluid resuscitation in patients with hypotension caused by hypovolemia can achieve an adequate response. However, it is often harmful in cases where the hypotension is not hypovolemia-related [3,4]. Therefore, the volume status should be evaluated as an initial step in critically ill patients in the ED. There are several invasive methods for assessing volume status in mechanically ventilated patients under sedation in intensive care settings [5]. However, a majority of these methods are not feasible for spontaneously breathing patients in the ED [6]. Therefore, there is a need for fast, easy, and noninvasive methods for volume assessment in critically ill patients in the ED.
Photoplethysmography (PPG) is an optical technology employed in pulse oximeters. The perfusion index is the ratio between PPG signals of the pulsatile (arterial component) and non-pulsatile components (other tissues) in the pulse oximeter [7]. The pleth variability index (PVI) is a dynamic indicator of the volume status, which can be determined by respiratory variations in the perfusion index [8]. Previous studies have assessed the ability of the PVI value to predict fluid responsiveness in mechanically ventilated patients [9]. However, the utility of the PVI in determining acute intravascular volume changes in spontaneously breathing patients remains unclear. Previous studies on the PVI in spontaneously breathing patients reported that it could weakly predict intravascular volume changes [8,10]. However, hypovolemia induced by acute blood depletion was present in only a small number of the patients included in these studies [10].
End-tidal carbon dioxide (EtCO2) is an important metabolism indicator that could be used to assess various conditions of critically ill patients, including volume status and responsiveness [11,12]. EtCO2 utility in noninvasive monitoring of patients in shock has been reported [13]; however, there have been few studies on EtCO2 accuracy in early-stage hypovolemia [14,15].
Previous studies have reported the role of the vena cava collapsibility index (VCCI) in volume status prediction [16]. The VCCI has been demonstrated as an indirect indicator of central venous pressure with respect to volume status [5,6]. However, its predictive ability of early phase acute volume depletion remains unclear [17]. Further, the mean arterial pressure (MAP) and the pulse pressure (PP) are widely used noninvasive monitoring indexes derived from the arterial blood pressure (BP). Although these indexes have been suggested in hypovolemia monitoring [18], their utility in the advanced shock stages remains unclear.
We aimed to investigate the utility of noninvasive volume assessment methods, including PVI, EtCO2, VCCI, MAP, and PP, in monitoring acute intravascular volume changes in healthy adults after 1-unit (450 mL) blood donation and passive leg raise (PLR).
METHODS
Study design and setting
This prospective observational study was conducted in the blood center of an academic hospital that received an annual blood donation from 17,000 healthy volunteers (male 96.4%) between June 2015 and July 2015. Institutional review board approval was obtained for the study (08.07.2014-15/12). All study participants provided written informed consent prior to study participation.
Participant selection
We enrolled all healthy blood donors who provided consent for study participation. We excluded volunteers unsuitable for blood donation (active infection, intravenous drug use, hemoglobin levels <13 g/dL, current medication use, alcohol use within the last 24 hours). Moreover, we excluded volunteers with cardiac dysrhythmia or skin disruption, which may affect the study measurements.
Study protocol
We measured the PVI, EtCO2, VCCI, MAP, and PP with the patient in supine position before donation and at the 0th and 10th minute postdonation. Subsequently, the patients performed the 45-degree PLR (Fig. 1), and the aforementioned measurements were obtained after holding this position for 90 seconds. We obtained PVI and SpO2 measurements using the Radical-7 PPG device (Masimo Corp., Irvine, CA, USA). A pulse oximeter probe (LNOP Adt, Masimo Corp.) was attached on the 4th finger of the non-dominant hand. The pulse oximeter was connected to the Radical 7 monitor (Masimo Corp.) with PVI software ver. 7.0.3.3. (Masimo Corp.).

Measurement positions. (A) Predonation, postdonation at the 0th and postdonation at the 10th minute measurements and (B) passive leg raise measurements.
We used the B-20 monitor (Mindray, Milwaukee, WI, USA) to measure the arterial BP, respiratory rate, and EtCO2 using the side-stream method. We conducted side-stream EtCO2 measurements using a sampling port adapted to a simple oxygen mask. MAP and PP were calculated as follows: PP=systolic BP-diastolic BP and MAP=2/3 diastolic BP+1/3 systolic BP.
VCCI measurements were obtained by an ultrasound-trained emergency physician using the Mylab Five (Esaote, Maastricht, the Netherlands) bedside ultrasound device and 1-8 MHz CA431 convex transducer.
Outcome measures
The primary outcomes were changes in PVI, EtCO2, MAP, VCCI, and PP at the 0th and 10th minute postdonation, as well as after the PLR.
Statistical analysis
We calculated the study sample size using G-Power for Mac OS X ver. 3.1.9.2 (Heinrich Heine University Dusseldorf, Dusseldorf, Germany). Considering four measurements from each volunteer, the estimated sample size was 30 with an assumed medium effect size of f=0.25, type-1 error of 0.05, and study power of 90%. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA) and Matlab ver. R2019a (Mathworks, Natick, MA, USA). The Shapiro-Wilk test was used to assess the normality of the variables. Normally distributed data were expressed as mean with standard deviation and 95% confidence interval (CI). Non-normally distributed data were expressed as median and interquartile range. Repeated-measures analysis of variance and Bonferroni-adjusted post hoc pairwise comparison were used to assess variations in the continuous variables. The Mauchly test was used to assess the validity of sphericity. We used Greenhouse-Geisser and Huynh-Feldt corrections if the epsilon was <0.75 and >0.75, respectively. Decimals in the results were rounded off. Statistical significance was set at P<0.05. For measurements that were assessed using the Bonferroni-adjusted post hoc test, statistical significance was set at P<0.008.
RESULTS
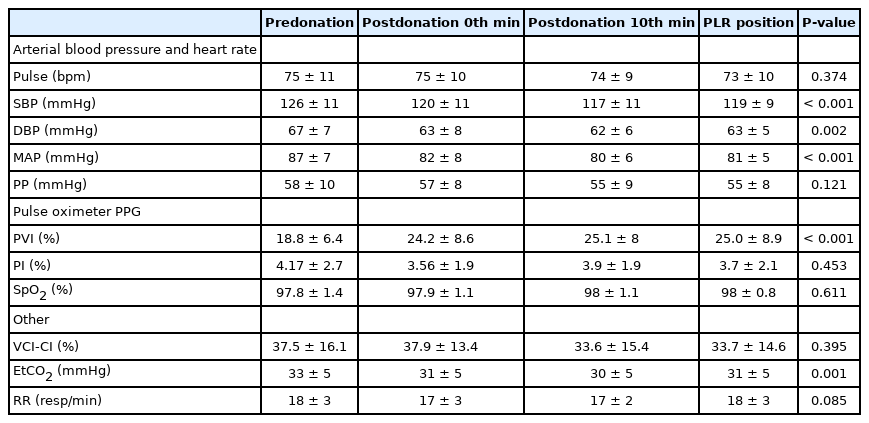
We enrolled 30 volunteers with a median age of 30 (6) years and a median weight of 75 (10) kilograms. All the study participants were male since the vast majority of the blood donors were male (96.4%). Table 1 shows changes in the hemodynamic, plethysmographic, and respiratory variables.
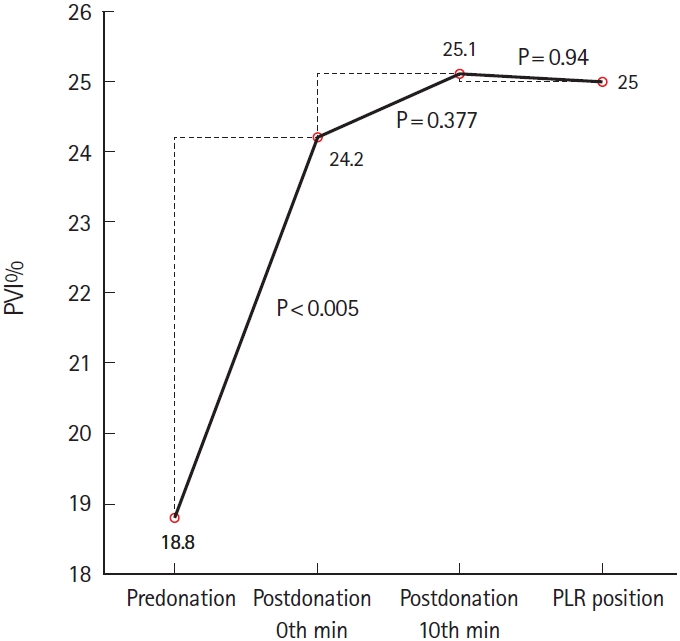
There were significant differences among all four PVI measurements (F[2.520;73.070]=10.162, P<0.001). Post hoc analysis revealed difference in the PVI at predonation (18.8%) and at the 0th minute postdonation (24.2%) (mean difference [MD], 5.4±5.9; 95% CI, -7.6 to -3.1; P<0.001) (Fig. 2).
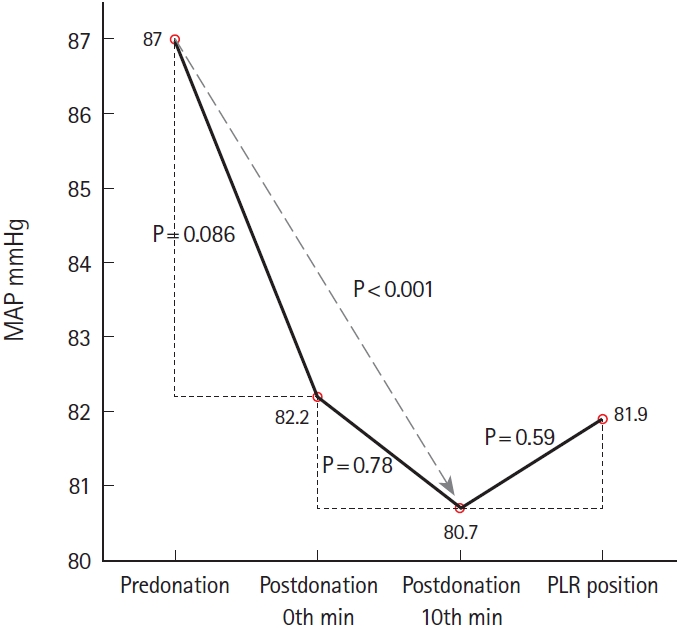
Further, there were significant changes in the mean values of EtCO2 and MAP: (F[2.503;72.577]=6.615; P<0.001) and (F[1.960; 56.832]=10.050; P<0.001), respectively. Post hoc analysis revealed a significant decrease in the EtCO2 at the 0th minute postdonation compared to the predonation value (MD, 2.4±4.6; 95% CI, 0.019 to 4.84; P=0.008). Moreover, there was a significant increase in the EtCO2 obtained in the PLR position compared with that obtained at the 10th min postdonation (MD, 1.8±3.2, 95% CI, 0.074 to 4.44, P=0.006) (Fig. 3). Compared with the predonation value, the MAP significantly decreased at the 10th minute postdonation (MD, 6.4±6.4; 95% CI, 3 to 9.7; P<0.001) (Fig. 4). There were no significant changes in the VCCI and PP values (P=1.0 for both values).
The change directions of the EtCO2 and MAP were consistent for all measurements (Figs. 2, 3). Further, the change direction of the PVI was consistently opposed to that of the EtCO2 and MAP (Fig. 1). However, there was no significant correlation between the PVI and EtCO2 changes between predonation and the 0th minute postdonation (r=-0.168; P=0.375; 95% CI, -0.447 to 0.157) and between the 10th minute postdonation and PLR (r=-0.218; P=0.246; 95% CI, -0.563 to 0.246). Table 2 presents the number of patients showing a consistent change direction of the PVI, EtCO2, and MAP.
DISCUSSION
We observed that a 450-mL acute blood depletion led to a significant change in the PVI, EtCO2, and MAP. Moreover, there was a significant increase in EtCO2 in the PLR position. To our knowledge, this is the first study to report PVI changes with acute intravascular volume depletion in spontaneously breathing volunteers.
Previous studies have assessed the volume status and responsiveness in mechanically ventilated patients and have reported that dynamic indicators, including the PVI based on cardiopulmonary interactions, are the best guides [19,20]. Although the utility of the PVI has been demonstrated in mechanically ventilated patients, its reliability in spontaneously breathing patients remains unclear [8,21]. A recent study by Demirci et al. [8] assessed PVI variations in the supine, Trendelenburg, and PLR positions in healthy spontaneously breathing volunteers. Although there was a significant change in the PVI, inconsistent change directions of the PVI indicated that it was not a reliable predictor of volume status change in those individuals. Schoonjans et al. [10] assessed changes in the PVI and PP in hypovolemia related to hemodialysis and blood donation, as well as after PLR. There was a significant posthypovolemia change in the PVI (22% vs. 18%, P=0.03); however, there was no significant post-PLR PP variation. Similarly, we observed a significant increase in the PVI after 450 mL blood loss; however, there was no significant change in consecutive PVI measurements (postdonation 10th minute and after PLR). There was a postdonation increase in the PVI of 24 (80%) volunteers. This was consistent with the change direction of PVI in the entire study cohort. However, there were insignificant post-PLR changes in the PVI. Similar to the findings by Demirci et al. [8], we observed an inconsistent post-PLR change in direction. This could be attributed to the fact that the volume expansion maneuvers, including PLR or Trendelenburg, could provide a fast transient change in the intravascular compartment without a significant change at the extravascular tissue level. Therefore, the PVI can better detect microcirculation changes at the tissue level after actual intravascular volume depletion.
Young et al. [22] assessed the predictive ability of EtCO2 variation for volume responsiveness after PLR or 500 mL intravenous fluid bolus in mechanically ventilated patients in the intensive care unit. There was a significant increase in the delta EtCO2 in the volume responded group (5.9±7.6% vs. 1.4±4.4%, P=0.02). A similar study by Arango-Granados et al. [12] evaluated the predictive ability of delta EtCO2 for PLR-induced volume responsiveness in spontaneously breathing volunteers and reported a weak correlation of PLR-induced changes in cardiac output (CO) with delta EtCO2. In our study, we observed a significant postdonation and post-PLR decrease and increase, respectively, in the mean EtCO2. This finding could be explained by the Frank-Starling Law, which implies a close correlation of CO and cardiac preload [23]. Therefore, a decrease in the cardiac preload due to intravascular volume loss could result in a decreased CO, which negatively affects adequate blood flow in tissue. Consequently, impaired microcirculation at the tissue level decreases metabolism and, in turn, EtCO2. On the other hand, a post-PLR increase in the CO improves microcirculation, which improves tissue metabolism and increases EtCO2.
Previous studies have assessed the utility of ultrasonographic-determined diameters of the inferior vena cava is assessing both volume status and responsiveness [16]. However, inconsistent findings have rendered the reliability of VCCI, especially in spontaneously breathing population, unclear [24]. This inconsistency could be attributed to differences in the respiratory patterns in spontaneously breathing patients. Akilli et al. [17] studied the inferior vena cava diameter as a hemorrhagic shock marker in spontaneously breathing patients and found that it was more valuable than traditional shock parameters, including the shock index, base excess, or lactic acid. In our study, there was no significant postdonation and post-PLR change in the VCCI. Although the previous study reported that sonographic evaluation of the inferior vena cava could detect hemodynamic changes in patients with hemorrhagic shock, it might not be reliable in early stage shock.
Based on blood loss severity, hemorrhagic shock is graded as class 1 to 4 [25]. Class 1 shock is defined as minor blood loss (<750 mL), which is not associated with significant vital sign changes [26]. MAP and PP are derived from the arterial BP and are frequent targets for ensuring tissue perfusion [23]. However, they rarely are affected in early phase hypovolemia [25]. In our study, the volunteers can be considered as having postdonation class 1 shock. Although we observed a significant change in the systolic BP, class 1 shock does not usually involve a change in the systolic BP, MAP, heart rate, PP, and respiratory rate [25,26]. Our findings indicate that the PVI and EtCO2 could promptly detect acute 450 mL blood loss before the other vital parameters. Moreover, a majority of the population showed a consistent direction in the significant change. Therefore, PVI and EtCO2 could be paramount to monitoring patients with acute blood loss who have normal vital parameters.
This study has several limitations. First, our participants were all male since the majority of the blood donors in our center were male (96.5%). Regarding critically ill patients in the ED, the heterogeneity of the study population limits the generalizability of the findings. For example, the pulse oximeter PPG waveform could be affected by systemic vascular resistance. Therefore, clinical conditions that affect systemic vascular resistance, including distributive shock or vasoactive drug use, might affect the reliability of PVI. Second, all our study participants had a hemoglobin level of >13 g/dL. Therefore, our findings might be inapplicable to patients with anemia. Third, the utility of PVI in spontaneously breathing patients has not been validated by studies with larger study cohorts. Most previous studies on PVI have assessed mechanically ventilated patients with constant minute ventilation. Therefore, the reliability of PVI in spontaneously ventilating patients with varying respiratory patterns remains unclear. Nevertheless, since there is a relatively regular respiratory pattern in healthy volunteers, our findings increase the credibility of PVI utility in healthy spontaneously breathing individuals.
In conclusion, our findings indicate that the PVI and EtCO2 can detect hemodynamic changes after acute 450 mL blood loss in healthy volunteers. The change in direction was inversely consistent between PVI and EtCO2. However, their ability to guide volume status in spontaneously breathing patients remains unclear.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
The benefit of PVI, EtCO2, VCCI, MAP, and PP in detecting acute intravascular volume changes in mechanically ventilated patients was demonstrated. However, previous data on the accuracy of these methods in spontaneously breathing patients is limited.
What is new in the current study
PVI, EtCO2, and MAP are reliable methods for detecting acute blood loss in spontaneously breathing population.