Dexmedetomidine as an adjunctive treatment for acute asthma
Article information
Abstract
Objective
This study aimed to compare the efficacy of using dexmedetomidine with salbutamol and salbutamol nebulization alone in patients with acute exacerbation of asthma presenting to the emergency department.
Methods
This clinical trial included 60 patients, in the age range of 18 to 55 years, with signs of bronchospasm presenting to the emergency department. In the intervention group, dexmedetomidine 0.5 µg/kg was injected intravenously and three doses of salbutamol nebulization were administered over 60 minutes. In the control group, salbutamol nebulization was administered for 60 minutes three times. The patient’s clinical status, based on clinical symptoms, consciousness, speech, breathing rate, heart rate, and blood pressure were recorded before the intervention, and peak expiratory flow rate and forced expiratory volume in 1 second were measured at 20, 40, and 60 minutes after intervention. Patients who did not respond to the intervention were excluded from the study within 60 minutes.
Results
The increased mean forced expiratory volume in 1 second and mean peak expiratory flow rate were found to be similar in both groups during the treatment (P=0.304). The mean systolic and diastolic blood pressure recorded at 40 and 60 minutes were significantly lower in the intervention group. During this study, no patient was excluded before 60 minutes.
Conclusion
Administration of dexmedetomidine in addition to standard salbutamol treatment has no beneficial effect in patients with acute asthma attacks and merely causes hypotension in patients.
INTRODUCTION
Chronic lung diseases are a common cause of disability and death worldwide. Asthma is one of the most common respiratory disorders contributing to several deaths annually [1]. It is reported that, globally, around 35.3% of people have asthma and at least 25% of the annual emergency visits to hospitals are associated with asthma [2]. Factors affecting asthma involve heredity, urbanization, air pollution, exposure to cigarette smoke, and unhealthy food habits [3].
Recurrent asthma is characterized by airway hypersensitivity, mucosal edema, and inflammation in the airways [4]. Medications used in asthma are bronchodilators (provide immediate relief of symptoms by relaxing the smooth muscle lining of the bronchi) and inhaled steroids (inhibit the underlying inflammatory process) [5]. Bronchodilators are beta-agonists with no effect on the underlying inflammation [6]. The most effective treatment for acute exacerbation of asthma is a short-acting inhaled beta-agonist (salbutamol) administered through a nebulizer, spray, or spacer [7].
It has been recently reported that the use of dexmedetomidine, with its sedative effect, could play a role in faster and more effective treatment of an acute attack [8]. Dexmedetomidine, a new drug belonging to the benzodiazepine family, has minimal respiratory depression effects unlike other benzodiazepines, and with its sedation properties, reduces the patient’s agitation during acute asthma, thereby lowering the respiratory distress and leading to faster recovery [9]. It is a central agonist of the alpha-adrenoreceptor that activates protein G in the locus coeruleus of the brainstem. It has a calming effect and reduces the release of norepinephrine [10]. This, in turn, will reduce the respiratory distress and lower the blood pressure, heart rate and respiratory rate (RR). The half-life of this drug in the body ranges from 6 minutes to 2 hours; the short half-life helps in lessening the side effects [9,10]. Inhaled corticosteroids are used as first-line treatment for patients with persistent asthma, whose symptoms cannot be controlled with short-acting beta-agonists alone [11]. The goals in asthma treatment are to prevent symptoms, restore normal lung function, help the patient regain normal activity, prevent recurrences, provide an optimal drug regimen with minimal side effects, and ensure patient and family satisfaction [12].
The purpose of this study was to compare the efficacy of using dexmedetomidine with salbutamol and salbutamol nebulization alone in patients with acute asthma referred to the emergency department, and determine the extent of improvement in respiratory distress when using this treatment.
METHODS
Study design
This was a clinical trial including patients with signs of bronchospasm who presented to the emergency department of our hospital. The study was registered with the IRCT clinical trial registry (IRCT20131118015446N14), and received approval from Ahvaz Jundishapur University of Medical Sciences ethical committee (IR. AJUMS. REC.1397.021). Informed consent was obtained in written form from the enrolled patients.
Participants
Inclusion criteria
Patients in the age range of 18 to 55 years with acute asthma attack were included after obtaining their consent for participation in the study. Patients had a history of asthma or were confirmed by spirometry within 2 weeks of having a diagnosis of asthma.
Exclusion criteria
The exclusion criteria were as follows: dissatisfaction with the study, failure to respond to treatment, worsening clinical status requiring additional treatment such as magnesium sulfate, injectable epinephrine or intubation, use of intermittent positive pressure, and patient refusal to cooperate. Further, patients with underlying diseases having asthma-like manifestations, chronic bronchitis, coronary heart disease, cardiac arrhythmias, smokers with a history of smoking 10 packs/year or more, and use of beta-agonist nebulizer 6 hours before visiting the ED were also excluded [13].
Interventions
Patients were randomly divided into the intervention and the control groups. History and clinical examination of patients, state of consciousness, use of lateral respiratory muscles, wheezing, and parameters including RR, pulmonary function, blood pressure, and oxygen saturation were evaluated prior to the intervention.
In the intervention group, 0.5 µg/kg of dexmedetomidine was injected intravenously and salbutamol nebulization was administered three times over 60 minutes. Also, in the control group, salbutamol nebulization was administered three times over 60 minutes.
After measuring the peak expiratory flow rate (PEFR) and forced expiratory volume in 1 second (FEV1), the patient’s clinical status was assessed before the intervention, and at 20, 40, and 60 minutes after the intervention, based on clinical symptoms, consciousness, speech, breathing rate, heart rate, and blood pressure. Blood and pulse oxygen saturation percentages were also recorded at these intervals.
Randomization
Eligible patients were randomly divided into two groups such that there were an equal number of patients in both groups. Researchers were blinded in assigning individuals to the groups. This division was done using four permutation blocks.
Sample size
This was a phase II clinical study with a sample size of 50 patients, the minimum sample size required for such a study as per literature [14].
Statistical methods
All analyses were performed using IBM SPSS Statistics ver. 22 (IBM Corp., Armonk, NY, USA). Descriptive data were analyzed with mean ±standard deviation. Quantitative data between the two groups were compared using the independent t-test and Mann-Whitney test, based on the normality of the data. The level of statistical significance was considered to be less than 0.05.
RESULTS
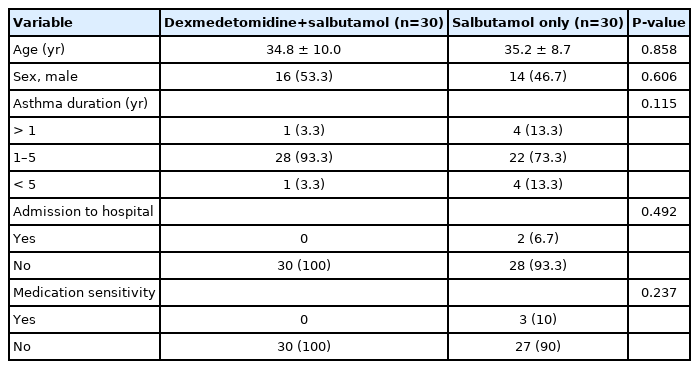
A total of 60 patients with an acute attack of asthma were enrolled (30 patients received intravenous dexmedetomidine and salbutamol nebulization, and 30 patients received salbutamol nebulization alone). Of them, 30 patients (50%) were male and the mean age of the patients was 35.2±9.29 years (Table 1). Increases in mean FEV1 (P=0.304) and mean PEFR (P=0.618) were found to be similar in both groups during treatment (Table 2). There was no statistically significant difference in the mean pulse rate of patients before the intervention and during the first hour (P<0.001). The mean RR in the patients during the first hour was not significantly different, and only a slight difference was seen before the intervention (Table 2).
The mean systolic blood pressure recorded at 40 and 60 minutes was significantly lower in the intervention group than in the control group (P=0.012). However, at baseline, the blood pressure was significantly higher in this group than in the control group (P=0.036). The mean diastolic blood pressure from 40 to 60 minutes was significantly lower in the intervention group than in the control group (P=0.007), while at baseline, it was significantly higher in the control group (P=0.040). Mean arterial blood oxygen levels were not significantly different in the patients before the intervention and during the first hour (P=0.640). No patient was excluded from the study before 60 minutes (Table 2).
DISCUSSION
This study aimed to compare the effect of injectable dexmedetomidine with nebulized salbutamol versus nebulized salbutamol alone in the treatment of acute asthma. In this study, patients with acute asthma attack were enrolled; patients were treated with dexmedetomidine and salbutamol nebulization or with salbutamol nebulization alone.
A case study by Cozzi et al. [8], on two acute-care emergency patients, found that dexmedetomidine reduced agitation, thereby lowering respiratory distress and leading to better treatment tolerance. However, our study found that administration of dexmedetomidine along with salbutamol had no significant effect on the patients’ spirometry parameters and their RR. The reason for the contrasting results could be due to differences in the sample size, demographic characteristics, and the type of study (case report vs. randomized clinical trial).
In a study by Lee et al. [14] including patients with chronic obstructive pulmonary disease (COPD) undergoing lung cancer surgery, it was observed that dexmedetomidine improved their postoperative clinical status. Groeben et al. [15], in their study of dogs, showed that dexmedetomidine improved bronchoconstriction and increased the volume of the respiratory tract. However, our study showed that dexmedetomidine administration had no effect on the improvement of spirometry parameters and RR of patients.
Lang et al. [16] used dexmedetomidine in a study of patients undergoing intrathecal anesthesia, which improved the heart rate and prevented tachycardia in the drug-receiving group, and in two cases converted the atrial fibrillation rhythm to sinus rhythm. In our study, however, it was found that dexmedetomidine did not affect the patients’ heart rate.
A case report of a 91-year-old man with COPD on non-invasive ventilation states that dexmedetomidine facilitated non-invasive ventilation and reduced the hospital stay [17].
A phase II study by Venn et al. [18] concluded that the use of dexmedetomidine to perform rescue intubation in patients reduced the propofol dose, as well as causing a slight decrease in blood pressure, heart rate, and heart output volume, and did not cause any complications after discontinuation. Although the design of this study was not similar to ours, a reduction in blood pressure in patients receiving dexmedetomidine was seen in our study as well; however, we did not observe any effect on patients’ heart rate.
In a case report of an 11-year-old child with COPD undergoing carotid artery stenting, the use of dexmedetomidine reduced respiratory depression and decreased blood pressure and heart rate [19].
A study by Xuan et al. [20] aimed to investigate the bronchial effects of alpha 2-adrenoceptor agonists. Inhalational alpha 2-agonists decreased the rapid bronchial response to allergens while intensifying the bronchial reaction to histamine, thereby increasing the symptoms. Rilmenidine, a new derivative of oxazoline, has fewer central effects compared to clonidine. In patients with asthma, short-term administration results in moderate improvement of bronchial obstruction and only partial protective action against various nonspecific stimulants or allergens, slightly enhancing the beneficial effects of beta 2-agonists. However, in our study, dexmedetomidine, a type of alpha 2-adrenoceptor agonist, had no observed effect on the severity of asthma.
Our study has some limitations. While dexmedetomidine is a novel treatment option being considered for an acute asthma attack, there are no sufficient studies and clinical trials to compare with the results of our study. The small sample size and nonparametric statistical analysis are other limitations of the study.
In summary, this study demonstrated that the administration of dexmedetomidine along with salbutamol nebulization had no beneficial effect in acute attacks of asthma, and it also led to hypotension in the patients. Therefore, we recommend that dexmedetomidine should not be included in the treatment of acute asthma.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was financially supported by the Office of Vice-chancellor for Technology and Research Development of Jundishapur Medical Sciences University, Ahvaz, Southwest Iran as part of Hossein Alizadeh’s thesis under the research code APRD-9701.
References
Article information Continued
Notes
Capsule Summary
What is already known
The most effective treatment for acute exacerbation of asthma is a short-acting inhaled beta-agonist.
What is new in the current study
Administration of dexmedetomidine in addition to standard salbutamol treatment has no effect on the improvement of severity in patients with acute asthma attacks and merely causes hypotension in them.