Pain management in the emergency department: a clinical review
Article information
Abstract
Pain is one of the most common reasons for patients to visit the emergency department. The ever-growing research on emergency department analgesia has challenged the current practices with respect to the optimal analgesic regimen for acute musculoskeletal pain, safe and judicious opioid prescribing, appropriate utilization of non-opioid therapeutics, and non-pharmacological treatment modalities. This clinical review is set to provide evidence-based answers to these challenging questions.
INTRODUCTION
Pain is one of the most common reasons for patients to visit the emergency department (ED) [1]. Due to the extensive number of visits to the ED related to pain, emergency medicine (EM) physicians and midlevel providers should be experts in providing safe, effective, and timely pain management. Given the ongoing opioid epidemic across the country, EM clinicians are uniquely positioned to combat this crisis by broader utilization of non-opioid analgesia, thoughtful prescribing of parenteral and oral opioids in the ED and at discharge and identifying and treating patients with opioid use disorder in the ED [2]. The research related to ED analgesia has grown exponentially over the past 10 years, frequently challenging dogmatic approaches to pain and numerous current pain management practices.
This focused clinical review is set to provide evidence-based answers to the following questions: What is the optimal analgesic treatment for musculoskeletal (MSK) pain that includes ibuprofen, acetaminophen, and opioids? When are opioids indicated and which drug(s), dose(s), and routes of administration are preferred? What is the role of non-opioid alternatives for managing pain in the ED? Is over-the-counter topical lidocaine 4% patch as good as prescription 5% lidocaine patch? What non-pharmacological interventions alleviate pain in the ED?
WHAT IS THE OPTIMAL ANALGESIC TREATMENT FOR MSK PAIN?
Non-opioid therapeutic agents (acetaminophen and non-steroidal anti-inflammatory drug [NSAID]’s) and opioids are frequently administered in combinations in the ED and at discharge for patients with MSK and soft tissue injuries (STIs) pain due to synergistic effects on pain relief [2,3]. The efficacy of combination therapies and analgesic superiority of a single class has been challenged recently by numerous clinical trials. A combination of acetaminophen (1 g) and ibuprofen (400 mg) has been found to lack analgesic and functional superiority over ibuprofen alone in managing acute MSK pain and back pain [4,5]. Similarly, this combination was not better than paracetamol (acetaminophen) alone in ED patients with minor acute MSK injuries [6]. The ibuprofen/acetaminophen combination was found to be as effective as oxycodone/acetaminophen, hydrocodone/acetaminophen, and codeine/acetaminophen for short-term pain relief (up to 2 hours) in ED patients with acute MSK pain including fractures [7]. A head-to-head comparison of NSAID’s (valdecoxib) to an opioid/acetaminophen combination demonstrated similar pain relief for short-term analgesia (up to 60 minutes) in ED patients with acute MSK [8].
In patients with acute STIs (sprain, strain, or joint, ligament, tendon, or muscle contusion), NSAID’s provided similar analgesic efficacy to acetaminophen at 1 to 2 hours and at 2 to 3 days (high certainty evidence), and to opioids at one hour (moderate certainty evidence) and at 4 to 7 days (low-certainty evidence) [9]. Similarly, oral paracetamol (acetaminophen), ibuprofen or a combination of both resulted in similar analgesic efficacy at the initial 2 hours and in the first 3 days in ED patients with mild to moderate STI pain [10].
Based on available evidence, oral acetaminophen or ibuprofen administered alone are equally effective for initial pain management in the ED and up to 2 to 3 days post-discharge in ED patients presenting with acute MSK and STI painful conditions. Patients with acute fracture might require a short 2 to 3 day course of opioids.
WHEN ARE OPIOIDS INDICATED AND WHICH DRUG(S), DOSE(S), AND ROUTES OF ADMINISTRATION ARE PREFERRED?
Opioids exert their clinical analgesic effect by binding to the opioid receptors (mu, delta, kappa) in the brain, spinal cord, and peripheral nervous system [11]. Parenteral and oral opioids are effective in controlling a variety of acute painful conditions of moderate to severe intensity [11,12]. However, a balance between the benefits and harms related to opioids should be thoughtfully considered prior to initiating opioid therapy in the ED [3,12]. The current opioid epidemic has led to several challenges in opioid administration, including optimal opioid selection, dosing regimen, and route in the ED and at discharge [3,13].
Indications
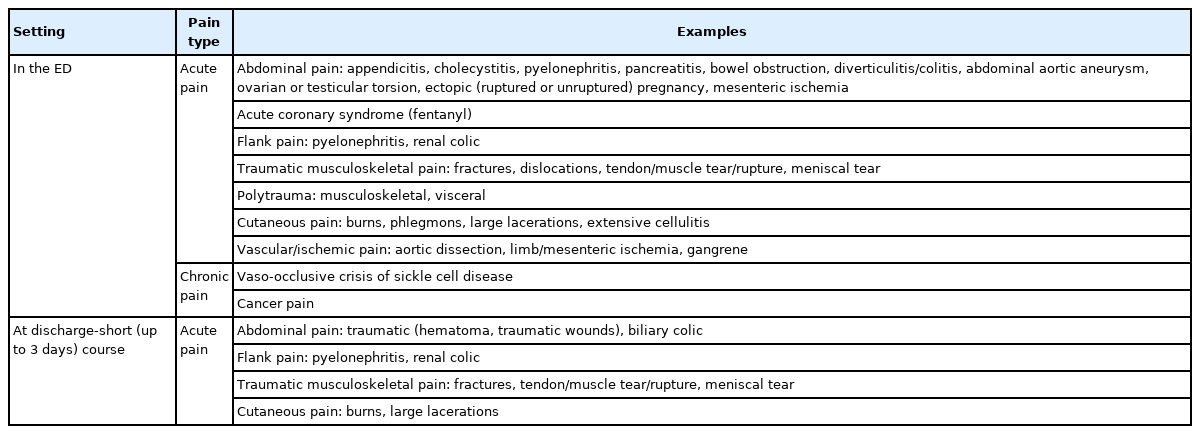
Opioid analgesics provide rapid and effective pain relief to patients presenting to the ED with a variety of acute painful syndromes, several chronic painful syndromes, and cancer-related pain syndromes (Table 1) [11]. Opioids should be used in the ED as a part of multimodal analgesia in conjunction with non-pharmacological and non-opioid therapies when the likelihood of their analgesic benefit is judged to exceed the likelihood of harm [12]. Opioids should not be used as first-line analgesics in the ED or at discharge in patients with acute back pain [14], acute headache [15-17], acute MSK pain (with the exception of fractures) [7], and acute dental pain [18] as the risks associated with their use (misuse, overdose, addiction) are significantly higher than the marginal, if any, pain relief provided.
Data supporting the use of opioids in the ED for treatment of acute exacerbation of chronic, non-cancer pain demonstrates higher likelihood of harm rather than benefit [19]. Opioid analgesics should not be routinely used in the ED for chronic non-cancer pain with a notable exception of vaso-occlusive crisis of sickle cell disease [20].
Choice of opioids
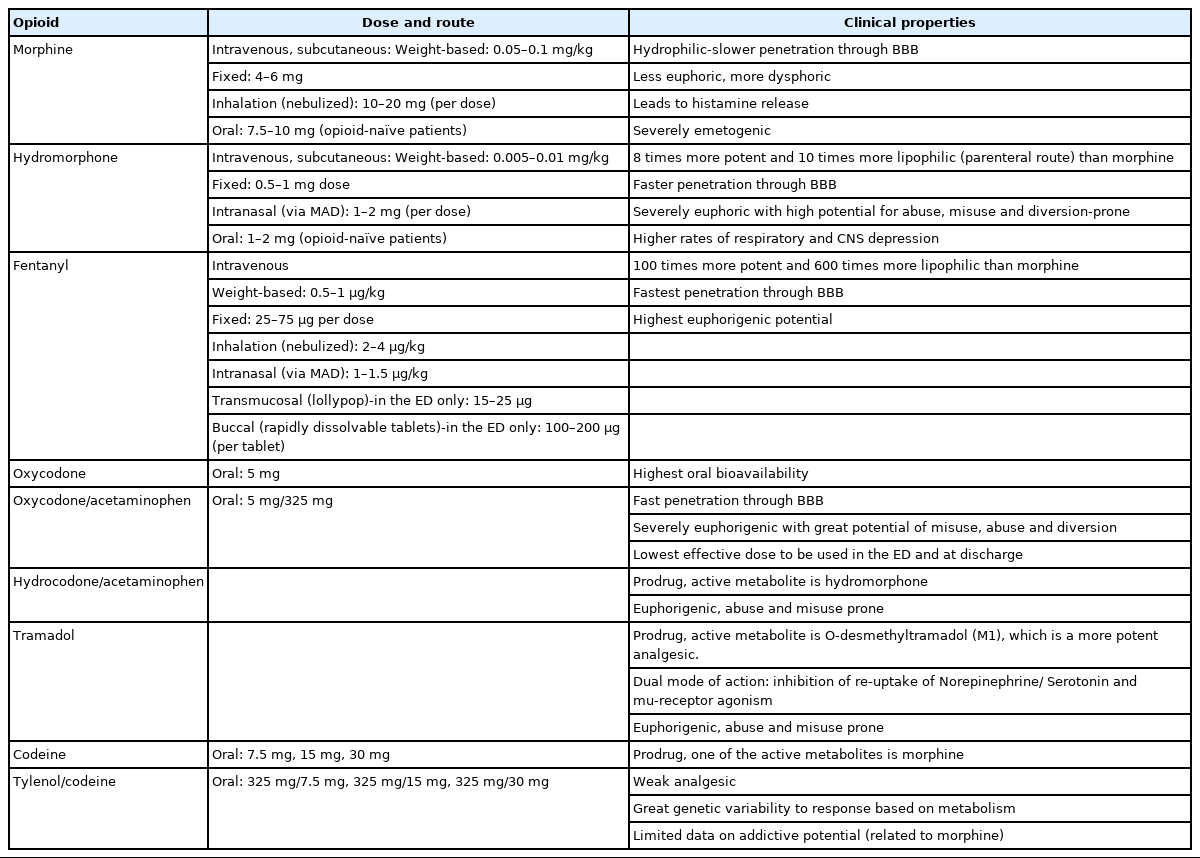
ED clinicians must recognize that commonly used opioids in the ED significantly differ from each other with respect to their ability to induce euphoria potentially leading to addiction (Table 2) [21]. Based on the available evidence, morphine sulfate administered either parenterally or orally in the ED and at discharge provides better balance of adequate analgesia and reduced euphoria and should be considered as the opioid of choice. In the situation when morphine is contraindicated and opioid analgesia is still warranted, parenteral fentanyl and oral hydrocodone are suitable alternatives in the ED and at discharge [11,12]. Parenteral and oral hydromorphone should be avoided as a first line opioid in the ED due to increased rates of respiratory and central nervous system depression (compared to morphine) as well as due to severe euphorigenic properties [22,23]. Oxycodone should not be used in the ED or at discharge due to greater potential for misuse, diversion, overdose, and the development of addiction with a lack of analgesic superiority to morphine and hydrocodone [21,24]. Similarly, tramadol should not be used in the ED and at discharge due to its modest, at best, analgesic efficacy, high potential for misuse, and host of numerous adverse effects (e.g., hypoglycemia, hyponatremia, seizures, serotonergic syndrome) [11,21]. Lastly, codeine plays no role in managing pain in the ED as it provides sub-optimal pain relief with significant genetic variability in analgesic response [11,21].
Dosing ranges and routes of administration
Pure mu-receptor agonists lack an analgesic ceiling, and their doses can be titrated upwards until pain is controlled, or side effects become intolerable or dangerous [11]. Parenteral opioid administration via an intravenous (IV) route achieves rapid, titratable, and effective pain relief in the ED and serves as a preferred route of opioid delivery [3,11]. When intravascular access is not readily available, ED clinicians should consider administration of opioids via intranasal (IN) route (fentanyl, hydromorphone), inhalation (via nebulizer) route (fentanyl, morphine), subcutaneous injection (morphine, hydromorphone), or transmucosal route (rapidly dissolvable fentanyl tablets) [3]. Intramuscular (IM) delivery of opioids in the ED should be avoided as it is associated with severe pain at the injection site, unpredictable absorption rates, soft tissue infection, and myofibrosis leading to a dose escalation and higher rates of adverse effects [3]. The oral route of opioid administration in the ED should be considered when feasible, even though it results in poor oral bioavailability (with the exception of oxycodone) and delayed onset of analgesia in the ED limiting its utility for rapid pain control [2,3,25].
WHAT IS THE ROLE OF NON-OPIOID ALTERNATIVES FOR MANAGING PAIN IN THE ED?
A variety of non-opioid alternatives have been broadly utilized in the ED for managing numerous painful syndromes with great success supported by a large body of literature.
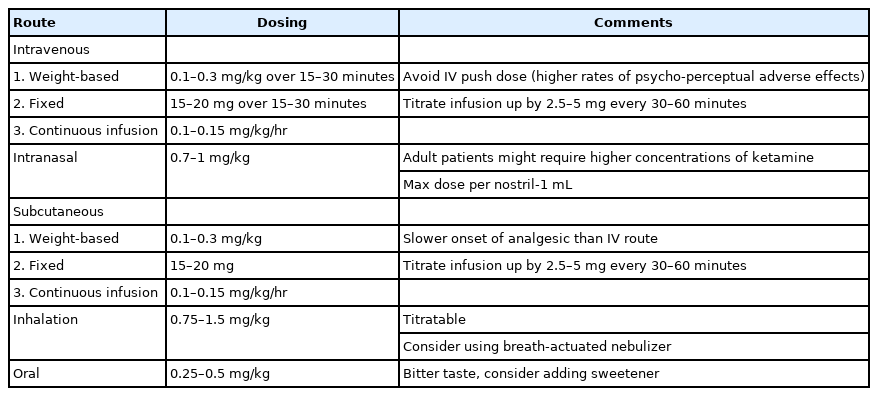
Ketamine is a non-competitive N-methyl-D-aspartate (NMDA)/glutamate receptor complex antagonist and potent analgesic suitable for the management of acute and chronic pain in the ED endorsed by the American College of Emergency Physicians and the American Academy of Emergency Medicine [2,26]. When administered in subdissociative (SDK) doses, the common IV dosing regimen is 0.1 to 0.3 mg/kg, or a 15 to 30 mg fixed dose administered over 15 minutes to reduce psycho-perceptual adverse effects [27-29]. SDK at 0.3 mg/kg IV has been shown to have similar efficacy in comparison to morphine 0.1 mg/kg IV for managing pain in the ED [28-31]. In the absence of IV access, SDK can be administered IN at 0.5 to 1 mg/kg with analgesia similar to IM and IN administration of opioids [32,33]. Additionally, nebulized ketamine at dosing range of 0.75 to 1.5 mg/kg was found to be effective in reducing acute pain in adult and pediatric ED patient with acute painful conditions [34]. Recently, a randomized clinical trial of 120 patients demonstrated similar analgesic efficacy of nebulized ketamine given at three different dosing regimens: 0.75, 1, and 1.5 mg/kg [35].
For chronic pain management, data on SDK is limited to case reports and case series. Ketamine can be a potential choice as part of an opioid sparing strategy in patients with tolerance or opioid dependence requiring management of acute or chronic pain management [12]. The current EM literature supports the administration of SDK as a safe and effective agent for use in ED pain management (Table 3).
Nitrous oxide
Nitrous oxide is a tasteless colorless gas administered in combination with oxygen via inhalation and is used as an anxiolytic, analgesic, and sedative agent. The mechanism of action involves NMDA receptor antagonism and release of endogenous opioid via opioid receptor agonism in the central nervous system [36]. Nitrous oxide is administered via facemask or nasal hood, is easily titratable, and has rapid onset and elimination making it an ideal agent for pain control in the ED [37]. The most common concentration is 50% to 70% nitrous oxide (30%–50% oxygen) via an on demand inhalation mechanism or continuous flow device [37,38]. Nitrous oxide is a potent, safe, effective inhalational anesthetic that provides quick and titratable pain relief for a variety of acutely painful complaints or procedures performed in the pediatric and adult ED population [37,38] (Table 4). Administration of nitrous oxide in concentrations higher than 70% or in combinations with opioids or benzodiazepines requires full cardiopulmonary monitoring. There are no fasting requirements or restrictions postadministration when nitrous oxide is given as a single agent in the ED [39].
Intravenous (IV) lidocaine
Lidocaine non-competitively blocks voltage-gated sodium channels as well as NMDA receptors and reduces hyperalgesia and central sensitization [40]. When administered IV at 1 to 1.5 mg/kg dose over 10 to 15 minutes, lidocaine causes minimal adverse effects (dizziness, tinnitus, periorbital and perioral numbness) that are transient and rapidly reversible [40,41]. Despite promising data from the earlier studies for renal colic [41], subsequent studies demonstrated analgesic inferiority of IV lidocaine to IV ketorolac alone and to IV ketorolac/lidocaine combination [42]. Similarly, IV lidocaine failed to demonstrate significant pain relief in ED patients presenting with acute headache [43], acute low back pain [44], and abdominal pain [45]. A recent systematic review found no definitive evidence to recommend IV lidocaine use and recommended further research within a larger and older population to assess the efficacy and safety in specific painful syndromes [46]. At present, IV lidocaine cannot be recommended for routine use in the ED and its administration should be based on a case by case basis.
Neuroleptics (antidopaminergic medications)
Haloperidol is a butyrophenone derivative that exerts its effects through dopamine receptor blockade (D2-R antagonist). Additionally, haloperidol binds to the histamine receptors, alpha-2 adrenergic receptors, 5HT-2 receptors and NMDA receptors and decreases hyperalgesia produced by chronic opioid use [47]. Droperidol is a butyrophenone derivative with potent dopamine D2 antagonist actions with additional actions such as A2 adrenoceptor agonist and 5HT-3, muscarinic and nicotinic receptors antagonist [48].
Haloperidol and droperidol have been used in the ED as an adjunct in treatment of headache [49], abdominal pain associated with cannabinoid hyperemesis syndrome [50], gastroparesis and cyclic vomiting syndrome [51], and chronic pain not responsive to opioids [52]. Traditional dosing regimens and routes include haloperidol: 2.5–5 mg IV, 5–10 mg IM; droperidol: 1.25–2.5 mg IV, 2.5–5 mg IM.
Ultrasound-guided regional anesthesia
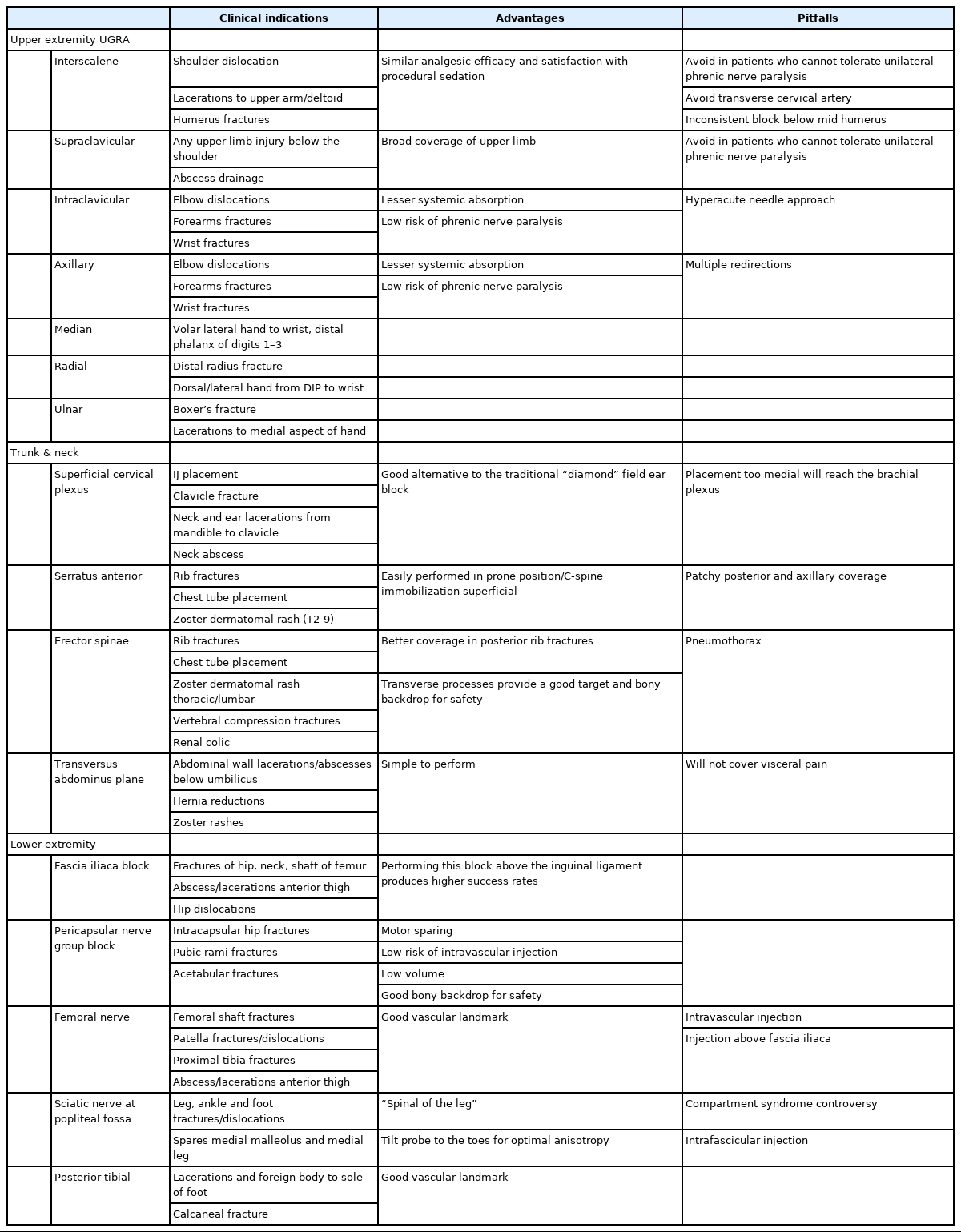
The most common ultrasound-guided regional anesthesia (UGRA) application in the ED is for management of patients presenting with hip/femur/upper extremity fractures followed by truncal and cervico-cranial applications (Table 5). UGRA provides significant pain reduction, alleviates the need for rescue opioid analgesia, and decreases the length of stay in the ED when compared to procedural sedation with no appreciable differences in analgesic efficacy and patient’s satisfaction. Furthermore, the utilization of UGRA in geriatric patients and patients with substance use disorder may eliminate any untoward side effects of parenteral opioid medications and reduce the dose of opioids [53,54]. Ultrasound guidance, calculation of maximum/lowest effective dose, aspiration before injection of 3 to 5 mL aliquots of local anesthetic of choice, and hydrolocation of structures with sterile saline at the start of infiltration are recommendations to prevent UGRA-related complications including local anesthetic systemic toxicity (LAST) [55].
An IV lipid emulsion (intralipid) therapy should be readily available when using UGRA to manage a LAST. Symptoms of LAST are typically progressive, from minor (tongue, perioral numbness, restlessness, muscle fasciculations, hypertension, tachycardia) to moderate (seizures, global central nervous system depression/confusion) to signs of impending cardiovascular collapse (bradycardia, conduction block, hypotension) [56].
If the patient is above 70 kg, an initial bolus of 100 mL 20% lipid emulsion should be administered over 2 to 3 minutes followed by a 20% lipid emulsion infusion of 200 to 250 mL over 15 to 20 minutes. For patients below 70 kg, the bolus dose is a weight-based 1.5 mL/kg followed by a 0.25 mL/kg/min infusion. If circulatory stability is not attained, rebolusing up to two further times and increasing the infusion to 0.5 mL/kg/min is suggested. The maximum recommended dose of lipid emulsion is 12 mL/kg [57].
IS OVER-THE-COUNTER TOPICAL LIDOCAINE 4% PATCH AS GOOD AS PRESCRIPTION 5% LIDOCAINE PATCH?
Topical lidocaine has been used in patients with herpetic neuralgia, diabetic polyneuropathy, osteoarthritis, and MSK pain including low back pain [58,59]. Its use is contraindicated in patients with hypersensitivity to amide anesthetics, open wounds, and skin eczema. The most common adverse effects include skin erythema, edema, and occasional burning at the application site [58,59]. The dosing regimens are 1 to 3 patches daily with a 12-hour free period with a maximum dose of three patches daily [58]. A 5% topical lidocaine plaster was found to be more effective than capsaicin, gabapentin, pregabalin, and placebo and with fewer adverse effects in patients with postherpetic neuralgia [60]. However, the cost of a pack of six patches that ranges from 45 to 150 US dollars in the US is prohibitive for the majority of patients [61,62]. In contrast, overthe-counter 4% lidocaine patch with average cost (pack of 6) of 6 to 12 US dollars might represent a suitable alternative as it was found to be non-inferior to the 5% patch with respect to efficacy, side effects, and impact on quality of life [61,62].
WHAT NON-PHARMACOLOGICAL INTERVENTIONS ALLEVIATE PAIN IN THE ED?
ED pain management for the most part heavily depends on pharmacological pain management where benefits of the pain relief must be carefully balanced against the adverse effects. Non-pharmacological pain management modalities are often effective in alleviating pain in the ED despite the limited number of studies with small sample sizes [63].
Cryotherapy
Multiple mechanisms have been proposed to explain the physiological basis for cryotherapy effectiveness, including inhibition of nociceptors, reducing the metabolic enzymatic activity of the injured tissue, and decreasing the nerve conduction velocity [64]. Cryotherapy is frequently used for managing acute MSK and soft tissue painful syndromes [65-68]. A common ED practice of applying a cold pack or ice pack to the skin with 10 minutes on, 10 minutes off can result in rapid analgesia in the ED and outpatient setting [66]. Another technique such as intensive targeted cryotherapy (wetted crushed ice in the plastic bag) was found to produce lower skin temperature than the application of the cold pack [67] and recently, has demonstrated more effective analgesia than chemical cold packs for acute MSK injuries in the ED [66]. Cryotherapy has been shown to be effective in the treatment of low back pain, neck pain, and a multitude of other sports-related injuries [66-69].
Heat therapy
When used in the context of a multimodal pain management, application of heat has shown moderate benefit in improving pain associated with acute neck and back strain in the ED [70]. Use of heat packs for treatment of chronic neck pain in the elderly population has been shown to decrease pain and improve range of motion [71]. Use of superficial heat was shown to be beneficial for the treatment of pain associated with temporomandibular disorders [72].
A systematic review provided tentative evidence that transcutaneous electrical nerve stimulation (TENS) provided mild to moderate improvement in acute pain (back pain, fractures, headache, MSK pain, and procedural pain) as a stand-alone treatment modality in adult patients. The evidence suffered from high risk of bias and inadequate sample sizes [73]. Specifically to the ED, in a single center pilot study, TENS was found to be effective (average pain relief by 40% from the baseline) in 99% of patients with a variety of acute and chronic painful conditions, and to result in functional improvement in 83% of patients [74]. At present, however, robust data is lacking to support widespread use of TENS in the ED setting.
Acupuncture
The evidence for acupuncture is markedly heterogeneous, with a dearth of large, well designed, randomized controlled trials primarily supporting its use for chronic painful syndromes (back pain, osteoarthritis, and headache) [75]. Data on utilization of acupuncture (battlefield acupuncture) in the ED is limited with preliminary case series and smaller pilot studies showing promising results for pain control [76] but with larger, randomized studies demonstrating markedly mixed outcomes [77,78] that do not support widespread use of acupuncture in the ED.
Osteopathic manipulative treatment
Osteopathic manipulative treatment (OMT) is therapeutic maneuvers employed by osteopathic physicians to address dysfunctions in the MSK, myofascial, lymphatic, vascular or neurological structures. Studies looking at the application of OMT in the ED demonstrate analgesic improvement in MSK painful syndrome, reduction in the amount of parenteral analgesia [79,80] and decrease in length of stay [81]. However, there is a significant lack of large, randomized controlled trials. OMT is reimbursable as a procedure via five distinct codes in the American Medical Associations Current Procedural Terminology, making the utilization of this low risk [79], non-pharmacologic intervention more palatable in the hands of an OMT trained emergency physicians.
CONCLUSION
ED clinicians have a great responsibility to alleviate pain by all available means in a timely, efficient, and safe manner. The improved knowledge and set of skills of ED clinicians in managing pain have led to broader utilization of non-pharmacological and non-opioid treatment modalities as well as refined and judicious use of opioids. ED clinicians are uniquely positioned to perfect patient-centered, pain syndrome-targeted analgesia by relying on and incorporating evidence-based pain management into their daily practice.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Pain is one of the most common reasons for patients to visit the emergency department (ED). Due to the extensive number of visits to the ED related to pain, emergency medicine physicians and mid-level providers should be experts in providing safe, effective, and timely pain management.
What is new in the current study
This focused clinical review is set to provide evidence-based answers to the following questions: What is the optimal analgesic treatment for musculoskeletal pain that includes ibuprofen, acetaminophen, and opioids? When are opioids indicated and which drug(s), dose(s), and routes of administration are preferred? What is the role of non-opioid alternatives for managing pain in the ED? Is over-the-counter topical lidocaine 4% patch as good as prescription 5% lidocaine patch? What non-pharmacological interventions alleviate pain in the ED?