Augmented-Medication CardioPulmonary Resuscitation (AMCPR) trial: a study protocol for a randomized controlled trial
Article information
Abstract
Objective
Clinical trials on demodynamic-directed cardiopulmonary resuscitation have been limited. The aim of this study is to investigate whether Augmented-Medication CardioPulmonary Resuscitation (AMCPR) would improve the odds of return of spontaneous circulation (ROSC) in patients with out-of-hospital cardiac arrest.
Methods
This is a double-blind, single-center, randomized placebo-controlled trial that will be conducted in the emergency department of a tertiary, university-affiliated hospital in Seoul, Korea. A total of 148 adult patients with nontraumatic, nonshockable, out-of-hospital cardiac arrest who have an initial diastolic blood pressure above 20 mmHg will be randomly assigned to two groups of 74 patients (a 1:1 ratio). Patients will receive an intravenous dose of 40 IU of vasopressin with epinephrine, or a placebo with epinephrine. The primary endpoint is a sustained ROSC (over 20 minutes). Secondary endpoints are enhanced diastolic blood pressure, end-tidal carbon dioxide levels, acidosis, and lactate levels during resuscitation.
Discussion
AMCPR is a trial about tailored medication for select patients during resuscitation. This is the first randomized control trial to identify patients who would benefit from vasopressin for achieving ROSC. This study will provide evidence about the effect of administration of vasopressin with epinephrine to increase ROSC rate.
Trial registration
ClinicalTrials.gov identifier: NCT03191240. Registered on June 19, 2017.
INTRODUCTION
Out-of-hospital cardiac arrest (OHCA) is a major public health burden contributing to morbidity and mortality worldwide [1-3]. A recent epidemiological study reported that over one in 10,000 patients suffered from cardiac arrest and survival rates were lower than 20% [4]. To improve outcomes, the guidelines of the American Heart Association and European Resuscitation Council have strengthened the chain of survival, including early recognition, effective chest compression, timely defibrillation, and prompt use of vasopressors [5-7]. Among various vasopressors, epinephrine and vasopressin have been considered as candidates to improve the chance of return of spontaneous circulation (ROSC) [8,9]. Because vasopressin stimulates smooth muscles to vasoconstrict without a catecholamine effect, it can be used for patients with cardiac arrest by utilizing a different mechanism than with epinephrine [10]. However, current guidelines do not recommended vasopressin as a replacement for epinephrine (class III) based on previous research that additional use of vasopressin did not have any outcome benefit compared to epinephrine alone [11-13]. However, previous controlled trials did not observe real-time changes in diastolic blood pressure or end-tidal carbon dioxide levels, which are considered surrogate markers of organ perfusion [11,12]. Moreover, the effectiveness of vasopressin could be masked especially in patient with cardiac arrest who had sufficient response to conventional epinephrine doses for increasing vital organ perfusion [13,14].
Although resuscitation guidelines remain uniform across all cardiac arrest patients, individualizing resuscitation to appropriate hemodynamic goals rather than using a standard “one-size-fits-all” cardiopulmonary resuscitation (CPR) seems a promising strategy in highly monitored patients. Previous randomized controlled animal studies established that hemodynamic-directed targeted CPR results in superior outcomes compared to standard CPR [15-17]. However clinical trials on hemodynamic-directed CPR have been limited. Therefore, individualization strategies, such as blood pressure-directed CPR including the administration of additional vasopressors in refractory cardiac arrest patients who cannot maintain diastolic blood pressure with epinephrine injection alone during CPR, may be useful.
The aim of the study is to determine whether the addition of vasopressin in refractory cardiac arrest patients during CPR improves ROSC rate. We therefore designed the Augmented-Medication CardioPulmonary Resuscitation (AMCPR) trial. We hypothesized that the addition of vasopressin when patients cannot achieve a diastolic blood pressure over 20 mmHg with epinephrine during CPR, would improve outcomes.
METHODS
Ethical statements
The trial protocol was approved by the Institutional Review Board of Asan Medical Center (No. 2017-0669) and the Ministry of Food and Drug Safety in Korea. The requirement for informed consent was waived by the Ethics Committee because of the emergent need for treatment in cardiac arrest. The legal representatives of the patients were later informed about the trial.
Study design
We will conduct a randomized, double-blind, placebo-controlled, single-center trial among patients with nontraumatic OHCA in the emergency department of an urban, tertiary hospital, located in Seoul, Korea. Figs. 1 and 2 show the study flow chart and the schedule, respectively. A SPIRIT (Standard Protocol Items: Recommendations for Clinical Interventional Trials) checklist is also available (Supplementary Material 1).
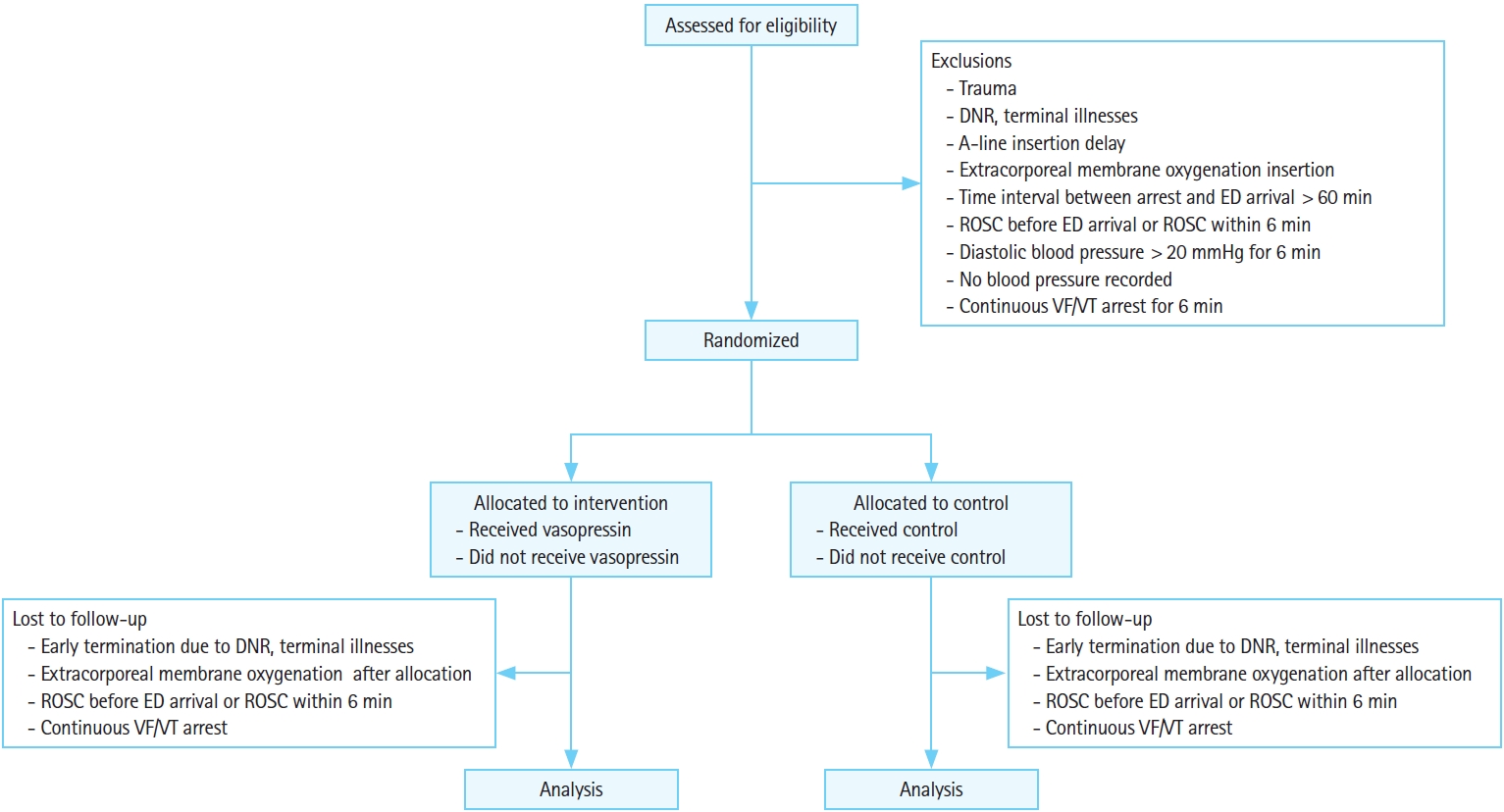
Trial flowchart. DNR, do not resuscitate; ED, emergency department; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
Eligibility criteria
Adult patients (above 18 years old) with nontraumatic, nonshockable OHCA and with diastolic blood pressure below 20 mmHg measured by invasive radial or femoral arterial line at presentation will be included. The following exclusion criteria apply: patients with traumatic cardiac arrest, patients with a signed donot-resuscitate order, patients with an underlying terminal-state disease without an active treatment plan, patients with failed arterial line insertion within 6 minutes, patients receiving extracorporeal membrane oxygenation, patients with a prehospital downtime longer than 60 minutes, patients with successful ROSC before hospital arrival or ROSC within 6 minutes, and patients with diastolic blood pressure above 20 mmHg for 6 minutes during resuscitation.
Randomization and study medication
The patients will be randomly assigned in a 1:1 ratio via a random number generator to either the intervention group or the placebo group. The trial administration nurse will open a premade, concealed, uniquely numbered, but otherwise identical-appearing card containing a treatment (i.e., vasopressin or placebo). Then, he/she will prepare the vasopressin (40 IU) or saline placebo. During the trial, all emergency physicians, nurses, and interns will be unaware of which drugs will be used. Unblinding will only take place in case of an unexpected serious adverse reaction. After randomization, patients will receive either 1 mg of epinephrine and 40 IU of vasopressin or 1 mg of epinephrine and saline placebo in separate injections less than 10 seconds apart. If ROSC is not achieved within the following 3 minutes, patients will be administered one more vasopressin (40 IU) or placebo with epinephrine injection. After that, only 1 mg of epinephrine will be administered every 3 minutes for both groups. Other conventional drugs, including amiodarone, calcium, or bicarbonate will also be administered at the clinician’s discretion and no other drugs will be administered.
Clinical management
Advanced cardiac life support will be administered in accordance with the last international guidelines and local procedures [5,6]. In brief, all cardiac arrests presenting to the emergency department will have resuscitation initiated while being assessed for eligibility. Defibrillation will be performed for patients with shockable rhythms. In eligible patients, an arterial line will be placed for continuous monitoring via the radial or femoral arteries. Confirmation of adequate placement of the catheter will be performed by an experienced emergency physician on duty using bedside ultrasonography. Moreover, an arterial line square wave test will be conducted to confirm adequate function of invasive catheters. Survivors who are successfully resuscitated will be admitted to intensive care units for further postcardiac arrest care, including targeted temperature management, percutaneous coronary intervention, mechanical ventilation, and renal replacement therapy.
Data and laboratory measurements
All data will be anonymized and collected according to Utstein guidelines using a database designed with Microsoft Access (Microsoft Corp., Redmond, WA, USA) by independent blinded researchers [18]. Baseline characteristics and other laboratory variables will be obtained from electronic medical records at the study facility. We will also collect prehospital information, including prehospital total no-flow and low-flow time, presence of shockable rhythm, administration time of epinephrine, and amount of epinephrine. In addition, arterial blood pressure and end-tidal carbon dioxide levels during resuscitation will be recorded on video and recorded in the electronic database every 10 seconds. Arterial blood gases will be performed initially, at 10 minutes, at 20 minutes, and at the end of resuscitation. All case-record data will be subsequently collected in a database, where a random sample of 10% of the data will be assessed by the data and safety monitoring committee.
Outcome measures
The primary outcome will be sustained ROSC defined as the spontaneous return of a palpable pulse and measurable blood pressure longer than 20 minutes. Secondary outcomes are (1) survival to discharge, (2) good neurologic recovery at discharge (Cerebral Performance Category 1 or 2) [19], (3) elevation of diastolic blood pressure, (4) elevation of end-tidal carbon dioxide levels, and (5) improvement of acidosis and lactate levels. Diastolic blood pressure and end-tidal carbon dioxide levels will be obtained every 10 seconds from a recording of the entire resuscitation period. Then, trends and median values will be compared between groups. Acidosis and lactate levels will be obtained from arterial catheters on initial placement and 10 and 20 minutes later.
Adverse events
Risks to participants in this study may be minimal. There are no previous reports about harmful effects of administration of vasopressin up to 80 IU for patients with OHCA during resuscitation. Therefore, we have no predefined adverse events for this trial.
Statistical analysis
Data analysis will be performed with the intention-to-treat set, the full analysis set, and the per-protocol analysis set. The sample size was calculated based on an expected difference of 25%. We assumed that 30% of patients in the control group would achieve ROSC. For the P-value of 0.05 and the statistical power of 0.80, a total sample size of 74 patients will be required in each group. Assuming a 15% dropout rate, we will enroll 174 patients. All collected data will be analyzed using descriptive methods according to the intervention and control group. For continuous variables, mean with standard deviation or median with interquartile range will be presented depending on normality. For binary variables, number with percentage will be reported. Analysis will employ the t-test or the Mann-Whitney U-test for continuous variables and the chi-square test or Fisher exact test for categorical variables. Statistical significance will be considered as a P-value less than 0.05. All statistical analysis will be conducted using IBM SPSS ver. 27.0 (IBM Corp., Armonk, NY, USA).
DISCUSSION
Vasopressors have been considered key elements for improving chance of ROSC for patients with out-of-hospital cardiac arrest. Despite vasopressin being more effective than epinephrine in animal studies, past meta-analysis of clinical studies showed no evident benefit of vasopressin over epinephrine in human CPR [20,21]. While the main focus of previous trials was universal administration of vasopressin during resuscitation, the key goal of this trial was to assess the effects of vasopressin on outcomes only among patients with cardiac arrest who cannot maintain diastolic blood pressure above 20 mmHg. Because vasopressin is another vasopressor which has a different mechanism from epinephrine, it may have a synergic effect with epinephrine for the patients in a severe vasoplegic state during CPR [22].
The current guidelines suggest monitoring diastolic blood pressures and improving the quality of resuscitation when the level of diastolic blood pressures is lower than 20 mmHg [5,6]. Although insertion of an arterial line during chest compressions can be technically difficult, it is worth attempting not only for hemodynamic-directed CPR but also for monitoring dynamic changes in acidbase metabolism [23,24]. Accessing the radial or femoral artery with catheters will be attempted and adequate placement and function of the catheters will be confirmed by bedside sonography and square wave test. Failed insertion of the arterial line is an exclusion criterion, and withdrawal due to failed arterial line insertion may be higher than expected. However, at least two experienced emergency physicians on duty will try to insert the catheter to reduce exclusion of cases.
The patients will receive 40 IU of vasopressin just after administration of 1 mg epinephrine and receive one more repeated dose of both if ROSC is not achieved. This dosage of vasopressin has been proven to be safe without serious adverse outcomes in past clinical trials [25]. Furthermore, we will exclude patients with shockable rhythms (i.e., ventricular fibrillation and ventricular tachycardia) because in those cases immediate defibrillation is more important than arterial catheterization, which is a time-dependent procedure in this trial.
Enrollment of the patients started from August 2017. Along with a recent trial with in-hospital cardiac arrest patients, this study will provide valuable evidence about the effectiveness of the addition of vasopressin to epinephrine among patients with refractory low organ perfusion pressure in resuscitation of OHCA. If this treatment is shown to be effective, the use of an arterial line for monitoring diastolic blood pressure and administration of additional vasopressors could be a promising treatment strategy for patients with OHCA.
SUPPLEMENTARY MATERIAL
Supplementary material is available at https://doi.org/10.15441/ceem.22.367.
Supplementary Material 1.
SPIRIT (Standard Protocol Items: Recommendations for Clinical Interventional Trials) checklist for trials.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This study was supported by a grant from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (No. 2017IT0669).
AUTHOR CONTRIBUTIONS
Conceptualization: WYK, SMR; Data curation: JK, YJK; Formal analysis: DKO, JK; Funding acquisition: WYK; Investigation: SMK, SIH; Methodology: DKO, JK, BC; Project administration: WYK; Visualization: JK, YJK, SMR, SMK, SIH, BC; Writing–original draft: DKO, JK; Writing–review & writing: YJK, SMR, WYK.
All authors read and approved the final manuscript.
References
Article information Continued
Notes
Capsule Summary
What is already known
A combination of vasopressin and epinephrine for patients with out-of-hospital cardiac arrest has shown not to improve outcomes.
What is new in the current study
We propose the Augmented-Medication CardioPulmonary Resuscitation (AMCPR) trial to determine whether the addition of vasopressin in refractory cardiac arrest patients with low diastolic blood pressure during cardiopulmonary resuscitation improves return of spontaneous circulation rate.

