The role of repeated brain computed tomography based on ultrasound monitoring of optic nerve sheath diameter after moderate traumatic brain injury
Article information
Abstract
Objective
This study was conducted to evaluate the association between changes in repeated brain computed tomography (CT) findings and the optic nerve sheath diameter (ONSD) determined by ocular ultrasonography in patients with moderate blunt traumatic brain injury (TBI).
Methods
This cross-sectional study was performed on patients with moderate blunt TBI (Glasgow Coma Scale, 9–12) who were referred to the emergency department during a 1-year period. Initially, all patients underwent a brain CT scan and primary ocular ultrasonography. Patients who were candidates for a second brain CT scan under observation in the emergency department also underwent a second ocular ultrasound. The primary outcome was the progression of brain lesions on repeated brain CT scans. Logistic regression and the area under receiver operating characteristic curve (AUC) were used.
Results
Overall, 204 patients with a mean age of 43±13.4 years were enrolled in the study. The study detected expanding changes in brain CT scans from 29 patients (14.2%). The progression of lesion on CT scan were significantly associated with changes in the Glasgow Coma Scale. In the second brain CT scan, there were significant associations between the progression of lesion on CT scan and the increased size of the ONSD measured on both axial and coronal sections (odds ratio, 17.3–47.5; AUC, 0.88–0.93).
Conclusion
Among patients with moderate TBI, an increase in ONSD on ocular ultrasound seems to be an appropriate criterion for repeating a brain CT scan to select a suitable therapeutic intervention.
INTRODUCTION
A brain computed tomography (CT) scan is the first diagnostic imaging procedure for patients with traumatic brain injury (TBI) admitted to the emergency department (ED). It is performed in all patients with moderate TBI. Under certain conditions, conducting a delayed brain CT scan is recommended to assess the progression of brain injury. However, delayed brain CT scans are not indicated in some cases and impose additional costs on the health system and expose patients to some complications. So, along with clinical criteria, it is necessary to develop an accessible, rapid, and safe diagnostic method for estimating the extent of TBIs [1,2]. The ocular ultrasound is used as a diagnostic method to determine intracranial pressure (ICP) by measuring optic nerve sheath diameter (ONSD) [3,4]. Several studies performed to evaluate the applicability of ocular ultrasound findings in assessing the severity of TBI have shown a significant positive correlation between ONSD and ICP [5,6]. Also, the ratio of ONSD to the transverse diameter of the eyeball in the ultrasound can be a significant predictor of changes in ICP [7]. Therefore, ONSD changes seem to be directly related to cerebrospinal fluid pressure changes. Studies have shown that patients with brain lesions and injuries usually have a larger ONSD than individuals without brain damage [8,9]. So, in patients with progressing or newly developed TBI, ONSD changes may indicate the need to repeat brain CT scans and consider therapeutic interventions as soon as possible. This study aimed to investigate the association between changes in repeated brain CT findings and the ONSD determined by ocular ultrasonography in patients with moderate blunt TBI.
METHODS
Ethical statements
This study was approved by the Ethics Committee of the Kerman University of Medical Sciences (No. IR.KMU.AH.REC.1399.180). Before patients entered the study, verbal consent was obtained from each patient’s medical proxy.
Study design
The study was a cross-sectional study on patients with moderate blunt TBI (Glasgow Coma Scale [GCS], 9–12 of 15) referred to the ED, a level II trauma center, during 1 year from March 1, 2020 to March 1, 2021. Inclusion criteria were age over 18 years, having blunt head trauma with a GCS between 9 and 12 (moderate TBI), and consent to participate from the patient’s medical proxy. Exclusion criteria were intubated patients, medical proxy not providing support for participation in the study, age <18 years, penetrating head trauma, body mass index over 30 kg/m2, pregnancy, having eyelid and orbital trauma and optic nerve injuries, emergency conditions requiring immediate transfer of the patient to the operating room, not repeating the brain CT scan, and ED arrival delayed by over 2 hours after injury. First, after arriving and having vital signs recorded, all patients underwent brain CT scans (Asteion 4, Toshiba). Patients then underwent a primary ocular ultrasound administered by an emergency medicine specialist at most 30 minutes after the CT scan. The providers were blinded to the results of the CT scans. The lesions observed on the brain CT scan and the baseline ONSD diameter, as well as other required information, were gathered by an emergency medicine assistant (postgraduate grade year 3) using a questionnaire. In patients who underwent a second brain CT scan based on a neurosurgeon’s counseling, a second ocular ultrasound was performed by the same emergency medicine specialist. According to the results, brain CT scans categorized patients into two groups: those with and without noticeable progression (expanding changes of intracranical lesions). Finally, changes in these two groups in ONSD and other variables were assessed.
Measurements
In both coronal and axial sections, a linear probe (7.5 MHz) with a standard technique of ultrasound machine (MX7, Mindray) was used to determine the ONSD. First, the patients were placed in a supine position. Then their closed eyelids were soaked with a sterile gel. A linear probe was placed transversely over the patient’s closed eyelid to obtain an axial view of the optic nerve. ONSD was measured at 3 mm posterior to the papilla. The probe was placed in a craniocaudal position to determine ONSD in the coronal view. The exact measurements were performed for both eyes [10].
Variables and outcomes
Age, sex, GCS, heart and respiratory rates, and ocular ultrasound findings (the ONSD of right and left eyes in both axial and coronal sections) were recorded. The primary outcome was the progression of brain lesions on repeated brain CT scans. We compared the variables according to the outcome.
Statistical analysis
Continuous variables were analyzed using the Student t-test, and categorical variables were analyzed using the chi-square test. For quantitative variables, mean±standard deviation was used. Odds ratios and 95% confidence intervals were used to express the association between the severity of the change of ONSD and an expanding change on the brain CT scan. Logistic regression was performed between brain CT scan and ONSD changes. Then the sensitivity, specificity, positive predictive value, negative predictive value, and the area under the curve (AUC) for predicting the need for repeating brain CT scans were calculated for ONSD. A P-value of <0.05 was considered statistically significant.
RESULTS
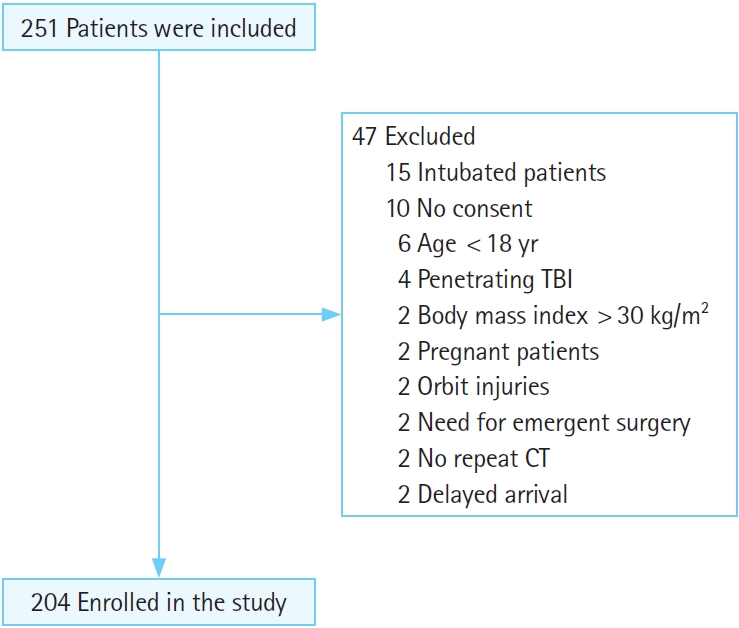
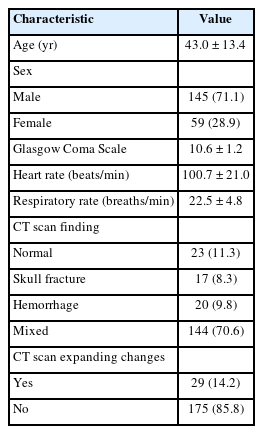
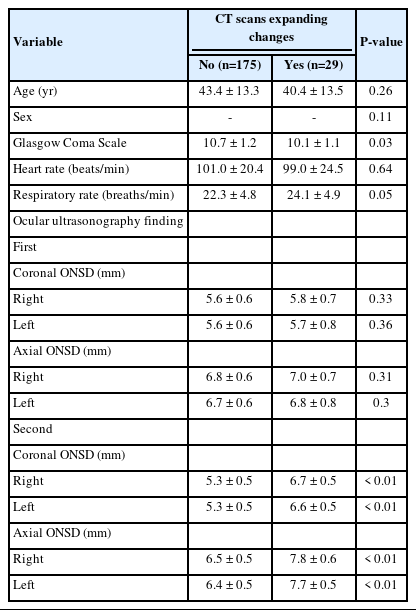
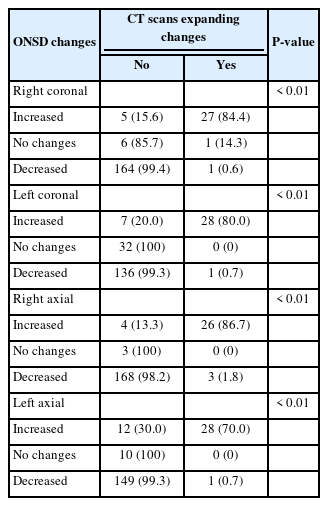
Overall, 251 patients with a diagnosis of moderate blunt TBI were admitted to the ED, of whom 47 were excluded, and finally, 204 patients were enrolled in the study (Fig. 1). The mean age of the patients was 43±13.4 years. Regarding sex, 145 patients (71.1%) were male, and 59 patients (28.9%) were female. Brain CT scan findings were normal in 23 patients (11.3%), 17 (8.3%) had skull fractures, 20 (9.8%) had a cerebral hemorrhage, and 144 (70.6%) had mixed lesions (more than one lesion such as intracranial hemorrhage, subdural hematoma, epidural hematoma, subarachnoid hemorrhage, contusion). Expanding changes in brain CT scans were observed in 29 patients (14.2%) (Table 1). The patients were divided into two groups: patients with and without brain CT scan expanding changes. Accordingly, there was a significant relationship between brain CT scan expanding changes and GCS (P=0.03); however, age, sex, respiratory, and heart rates were not significantly associated with brain CT scan expanding changes. Regarding the first ocular ultrasound, there was no significant relationship between changes in the brain CT scan and ONSD at axial and coronal sections in the right and left eyes. However, regarding the second brain CT scan, there were significant differences between the brain CT scan expanding changes with the increased size of the ONSD of the right and left eyes in both axial and coronal sections (P<0.01) (Tables 2, 3). In the univariate logistic regression analysis based on increased size of the ONSD, this significant difference was shown. As depicted, there were 47.5 times the odds of brain CT scan expanding changes in the patients with increased ONSD on the left coronal section. Other sonographic sections showing significant associations with changes on the brain CT are also noted (P<0.01) (Table 4). To determine the cutoff for the desired variables, we first performed logistic regression and then calculated the sensitivity and specificity for different cutoffs and selected the cutoff (0.5 mm) that had the highest sensitivity and specificity. Finally, both axial and coronal sections were used to calculate the sensitivity, specificity, positive predictive value, negative predictive value, and AUC of ONSD for the right and left eyes. The best results were related to the left coronal section for sensitivity, specificity, positive predictive value, negative predictive value, and AUC of ONSD (97.8%, 89.6%, 63.2%, 99.6%, 0.93, respectively) (Table 5).
DISCUSSION
This study investigated the value of ONSD obtained on ocular ultrasonography for the need to repeat a brain CT scan in patients with moderate blunt head TBI. Our results indicate that patients with a difference between the first and second ONSD might have a potential for the progression of the brain injury or the development of a new severe brain injury. Therefore, repeat head CT is recommended in these patients. Also, a decrease in GCS should be an indication for repeating the brain CT scan for these patients.
The optic nerve is part of the central nervous system. It is surrounded by the subarachnoid cerebrospinal fluid and dura mater. In the case of increased ICP, the size of the ONSD also increases. Ocular ultrasonography of the ONSD is a simple, noninvasive, and safe method for estimating cerebral ICP that can be an excellent alternative to invasive approaches. As a diagnostic method, ONSD measurement in patients with head trauma by ocular ultrasound was introduced years ago. Measurements are performed with a linear probe 3 mm behind the optic disc, in which the presence of a hypoechoic structure with a size of up to 5 mm is normal; nevertheless, a size above 5.7 mm indicates intracerebral hypertension [11]. Changes in ONSD correlate with ICP variations, so increased ICP (in conditions such as cerebral edema) is associated with elevated ONSD. On the other hand, ONSD decreases in situations where ICP is reduced (e.g., hyperventilation) [12,13]. Therefore, in patients with head trauma, the constant monitoring of ONSD by ocular ultrasonography can help monitor ICP and determine the prognosis of these patients. In one study, patients with reduced ONSD had a good prognosis and usually did not need surgery [14]. In addition to predicting ICP changes, ONSD dynamic changes may help predict bleeding volume and even the outcome in intracranial hemorrhage (ICH) patients. In their study, Skoloudik et al. [15] showed that the size of ONSD increases with bleeding volume in ICH patients. Their study showed that an ONSD size greater than 0.66 mm on an ocular ultrasound could predict a cerebral hemorrhage of more than 2.5 cm3, with a diagnostic accuracy of >90% [15]. In another study, Bender et al. [16] repeatedly measured ONSD by ocular ultrasound in patients with exacerbating clinical conditions, who often underwent brain CT scans. Their study showed that patients with elevated ONSD had worse outcomes. They also found significant relationships between elevated ONSD and decreased GCS and ICH bleeding volume [16]. Naldi et al. [17] also declared in their study that ICH patients had greater ONSD values than individuals without cerebral hemorrhage. Considering the correlation between ONSD and ICP changes, ocular ultrasound could be a reliable tool for monitoring patients with cerebral hemorrhage [9]. In our study, we also found a significant relationship between ONSD size in ocular ultrasound and either newly developed lesions or the progression of incremental lesions observed in brain CT scans. This means that the patients who showed signs of new injuries in the brain CT scan also had elevated ONSD on ocular ultrasound, suggesting the need to repeat the brain CT scan. Overall, ONSD monitoring in the ED can be beneficial for the rapid diagnosis of brain injury. In their study, Guzeldag et al. [18] showed a significant inverse correlation between ONSD and GCS, meaning that with decreasing GCS, there was an increase in the size of ONSD. Consistently, we also showed that the patients who developed new brain injuries or revealed deterioration in brain CT scans also had a decline in GCS, which could be a criterion for repeating the scan. Several studies in this field align with our research [19–21].
The limitations of this study include being a single-center study, not including patients with severe TBI, pregnant women, obese patients, and those under 18 years of age, and the fact that some patients’ medical proxies did not give consent for participation.
In conclusion, ocular ultrasonography of ONSD can help monitor patients with moderate blunt TBI in the ED. In these patients, increased ONSD on ocular ultrasound appears to be an appropriate criterion for repeating a brain CT scan and performing appropriate therapeutic interventions.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: AM, MT; Data curation: AM; Formal analysis: MM; Methodology: all authors; Resources: MT, AM; Software: MT, MM; Supervision: AM, MT; Visualization: AM, MT; Writing–original draft: MT; Writing–review & editing: MT, MM. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the Clinical Research Development Unit of Shahid Bahonar Hospital, Kerman University of Medical Sciences for their assistance.
References
Article information Continued
Notes
Capsule Summary
What is already known
Ocular ultrasound is used as a diagnostic method to determine intracranial pressure through measurement of the optic nerve sheath diameter (ONSD). Individuals with brain lesions and injuries usually have a larger ONSD than individuals without brain damage.
What is new in the current study
Ocular ultrasonography of ONSD can be helpful in monitoring patients with moderate traumatic brain injury. An increased ONSD in ocular ultrasound may be associated with progression of the brain injury or the development of a serious new brain injury. Increased ONSD on ocular ultrasound appears to be an appropriate criterion for repeating a brain computed tomography scan.