Benefits, key protocol components, and considerations for successful implementation of extracorporeal cardiopulmonary resuscitation: a review of the recent literature
Article information
Abstract
The application of venoarterial extracorporeal membrane oxygenation (ECMO) in patients unresponsive to conventional cardiopulmonary resuscitation (CPR) has significantly increased in recent years. To date, three published randomized trials have investigated the use of extracorporeal CPR (ECPR) in adults with refractory out-of-hospital cardiac arrest. Although these trials reported inconsistent results, they suggest that ECPR may have a significant survival benefit over conventional CPR in selected patients only when performed with strict protocol adherence in experienced emergency medical services–hospital systems. Several studies suggest that identifying suitable ECPR candidates and reducing the time from cardiac arrest to ECMO initiation are key to successful outcomes. Prehospital ECPR or the rendezvous approach may allow more patients to receive ECPR within acceptable timeframes than ECPR initiation on arrival at a capable hospital. ECPR is only one part of the system of care for resuscitation of cardiac arrest victims. Optimizing the chain of survival is critical to improving outcomes of patients receiving ECPR. Further studies are needed to find the optimal strategy for the use of ECPR.
INTRODUCTION
Cardiac arrest is a major cause of mortality worldwide, affecting 500,000 people annually in the United States alone [1–5]. In Korea, approximately 30,000 patients with cardiac arrest are treated using emergency medical services (EMS) each year [6]. Chances of survival after cardiac arrest decrease rapidly as the duration of cardiopulmonary resuscitation (CPR) increases [7–9]. Outcomes of patients’ refractory to initial resuscitation efforts are highly unfavorable [10,11].
The application of venoarterial extracorporeal membrane oxygenation (ECMO) in patients unresponsive to conventional CPR (commonly referred to as extracorporeal CPR [ECPR]) has significantly increased in recent years [12]. Cardiac output generated during conventional CPR is insufficient to maintain vital organ perfusion [13,14], and hypoxic-ischemic injury risk increases until the return of spontaneous circulation (ROSC). ECMO effectively restores vital organ perfusion, facilitating ROSC and buying time to identify and treat the underlying cause of cardiac arrest. Despite its increased use, the clinical efficacy of and the patient population most suitable for ECPR remain unclear. Although current resuscitation guidelines recommend ECPR as a rescue method in refractory cardiac arrest [15], a standard protocol for ECPR is lacking.
Here, we provide insights regarding the optimal implementation of ECPR based on a review of recent evidence on the clinical efficacy of ECPR and a summary of recent research on the key components of ECPR protocols and considerations for incorporating ECPR within a system of care.
IS ECPR MORE EFFECTIVE THAN CONVENTIONAL CPR?
A number of studies have investigated the relationships between ECPR and outcomes in patients with refractory cardiac arrest [16–19]. Although these studies report inconsistent results, several studies reported significant associations between ECPR use and positive outcomes [17,18]. To date, three published randomized trials have investigated ECPR use in adult patients with refractory cardiac arrest (Table 1) [20–22]. The first is the ARREST (Advanced Reperfusion Strategies for Refractory Cardiac Arrest) trial [20], which included adult patients with refractory ventricular fibrillation out-of-hospital cardiac arrest (OHCA) who had estimated transfer times to the University of Minnesota medical center of less than 30 minutes. The patients were randomized to either ECMO-facilitated resuscitation or standard advanced cardiovascular life support (ACLS) on arrival at the catheterization lab. The ARREST trial showed a significantly higher rate of survival to hospital discharge in the ECMO group compared to the standard ACLS group (43% vs. 7%) in the interim preplanned analysis after enrolling 30 patients, leading to premature termination of the trial because of the apparent survival benefit of ECMO-facilitated resuscitation.
The Prague OHCA study [21] was a single-center trial conducted in Prague, Czech Republic, which included 256 adult patients with refractory OHCA of presumed cardiac origin randomized during on-scene CPR to either an invasive strategy including prompt intra-arrest transport to a cardiac center under mechanical CPR, in-hospital ECPR, and immediate invasive assessment and treatment (n=124) or to the standard strategy (continued on-scene ACLS, n=132). In the study, the primary outcome was survival with a good neurologic outcome at 180 days, which was comparable between groups (31.5% vs. 22.0% for the invasive and standard strategies, respectively; P=0.09) in the intention-to-treat analysis, and the study was terminated based on a prespecified stopping rule for futility. However, in the Prague OHCA study, 66% of the invasive strategy group underwent ECPR compared to 8% of the standard strategy group. The crossovers could have affected the results of the intention-to-treat analysis. A secondary analysis of the Prague OHCA study revealed that the use of ECPR was significantly associated with 180-day survival in patients without prehospital ROSC [23]. Although both trials were terminated before enrolling the originally planned number of patients, both favored ECPR over conventional CPR. A meta-analysis by Scquizzato et al. [24] that included the two randomized trials and four propensity score–matched studies showed significant benefit of ECPR over conventional CPR with regard to survival with good neurologic outcomes.
The INCEPTION (Early Initiation of Extracorporeal Life Support in Refractory Out-of-Hospital Cardiac Arrest) trial [22] is the most recent randomized trial and involved 10 cardiosurgical centers served by 12 EMS agencies in the Netherlands. In this trial, 134 adult patients with witnessed refractory OHCA with initial shockable rhythm randomly received ECPR (n=70) or conventional CPR (n=64) at one of the participating centers. The authors found no significant between-group difference in 30-day survival with good neurologic outcomes (20% vs. 16% in the ECPR and conventional CPR groups, respectively; P=0.52).
In view of the inconsistent findings from the three randomized trials, whether ECPR can yield better outcomes than conventional CPR in patients with refractory OHCA remains unclear. However, considering that all three trials showed numerically higher survival rates for the ECPR groups, these trials may have been underpowered to detect any survival benefit that was present.
These three trials also had considerable differences in patient selection, trial setting, and EMS treatment strategy. The ARREST trial included only patients with shockable rhythms, randomized patients to treatment on arrival to the catheterization lab, and was performed at an experienced, high-volume center with a specific protocol. In contrast, 39.1% of patients in the Prague OHCA study presented with nonshockable rhythms. The randomization was performed during on-scene CPR, and thus patients assigned to the standard strategy group received continued on-scene resuscitation. The INCEPTION trial was performed at multiple centers with relatively low case volumes without a standardized ECPR protocol. The characteristics of the Prague OHCA study and INCEPTION trial could have contributed to the lower survival rates of ECPR groups and higher survival rates of conventional treatment groups in these trials compared to those in the ARREST trial, ultimately leading to the lack of significant survival benefit of ECPR seen in these two trials. Results of these trials suggest that ECPR may have a significant survival benefit over conventional CPR in selected patients only when performed in an experienced system with strict protocol adherence.
Given the greater accessibility to ECMO in in-hospital settings than out-of-hospital settings, patients with in-hospital cardiac arrest (IHCA) are likely better candidates for ECPR than those with OHCA. Although several observational studies have suggested survival benefits of ECPR in IHCA [7,25], results of randomized trials evaluating the effectiveness of ECPR in IHCA are currently lacking. Further studies are required to resolve the existing uncertainty over the benefits of ECPR.
KEY COMPONENTS OF THE ECPR PROTOCOL
The complete ECPR process must be well-defined through a rapidly deployable protocol to enable timely initiation of ECMO. ECPR outcomes in the absence of a well-defined ECPR protocol can be highly unfavorable [26–28]. Various protocols with differing candidate selection criteria, and cannulation and postcannulation managements are used for ECPR [29], none of which have been widely accepted.
Candidate selection
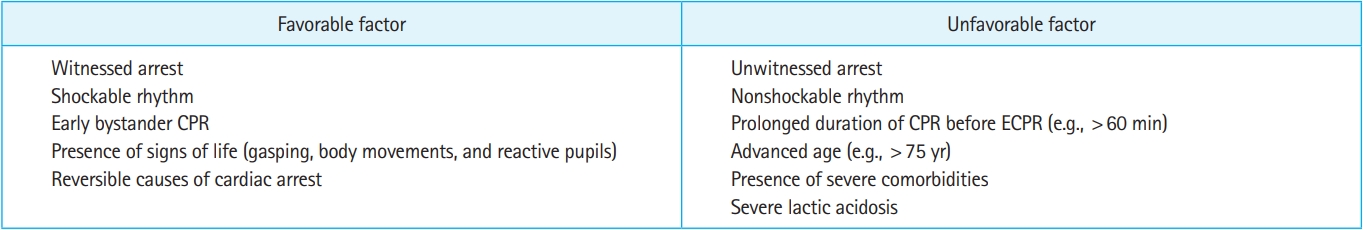
Clearly defined candidate selection criteria in an ECPR protocol would allow rapid recognition of potential candidates for ECPR. A variety of candidate selection criteria have been used in ECPR protocols [29,30]. Most are based on cardiac arrest-related factors known to be associated with outcomes of patients undergoing conventional CPR. Several factors, including witnessed arrest, presenting rhythm, and CPR duration, have been associated with outcomes after ECPR (Fig. 1) [23,31–37].
Advanced age has been included as an exclusion criterion in many protocols [29], although the cutoffs vary widely. Some studies reported significant associations between advanced age and poor outcomes in patients undergoing ECPR [38–41]; however, other studies reported no associations between advanced age and poor outcomes [32,33]. A recent study suggested that a significant number of elderly patients (>75 years) survive with good neurologic outcomes after ECPR [42]. ECPR should therefore not be excluded from treatment options based only on advanced age.
Initial electrocardiogram rhythm has frequently been used as an inclusion or exclusion criterion [29]. Several studies have shown significant associations between shockable rhythms and good outcomes in patients undergoing ECPR [31–36,43,44]. The survival rate in the invasive strategy group in the Prague OHCA study (31.5%) was lower than that in the ARREST trial (43.0%) that included only patients with initial shockable rhythms [20,21]. In the Prague OHCA study, the survival rate of patients with initial shockable rhythms in the invasive strategy group (48.6%) was close to that in the ARREST trial. In a post hoc analysis of the Prague OHCA study [43], initial shockable rhythm was significantly associated with neurologically favorable survival. Considering the results of these studies [20,21,31–36,43,44], only selecting patients with an initial shockable rhythm would improve outcomes after ECPR. Although patients with initial nonshockable rhythms have worse prognosis than those with initial shockable rhythms, patients with refractory cardiac arrest should not be excluded from ECPR only because of initial nonshockable rhythms. Several studies suggest that ECPR yields favorable outcomes in patients with initial nonshockable rhythms in the presence of other findings indicating likely good neurologic recovery (i.e., witnessed arrest, bystander CPR, and signs of life) [45,46]. In addition, a recent study reported that ECPR was associated with increased survival among patients whose electrocardiogram rhythm was initially nonshockable but later converted to a shockable rhythm [47].
The duration of CPR until ECPR initiation is a key determinant of outcomes after ECPR administration. Multiple studies suggest significant associations between prolonged CPR duration and poor outcomes after ECPR administration [36,48–50]. Defining refractory cardiac arrest with a shorter CPR duration in a protocol would lead to improved ECPR outcomes. Lamhaut et al. [26] reported an increase in survival from 8% to 29% after reducing the CPR duration for patient selection from 30 to 20 minutes. However, a shorter CPR duration may increase the risk of unnecessary exposure of patients who would survive with conventional CPR alone to ECMO. Although the maximal CPR duration beyond which ECPR becomes futile remains uncertain, 60 minutes is the most frequently used maximum allowable CPR duration [29]. Several studies suggest that the time to ECMO initiation of ≤60 minutes is associated with better outcomes [51,52]. The current Extracorporeal Life Support Organization (ELSO) guidelines also recommend commencing ECMO support within 60 minutes after cardiac arrest [53]. The time required to initiate ECMO support after the decision to apply ECPR may vary between institutions depending on the infrastructure and capabilities of the cannulation team. The mean time from catheterization lab arrival to ECMO initiation in the ARREST trial was 7 minutes [20], whereas the median time required for cannulation in the INCEPTION trial was 20 minutes [22]. Therefore, it is reasonable to set a maximal CPR duration in ECPR protocols, taking the time required for cannulation in each setting into account, with the goal of initiating ECMO within 60 minutes after cardiac arrest.
Clinical characteristics such as gasping, body movements, and reactive pupils, commonly referred to as signs of life, can help identify ECPR candidates. Signs of life have been associated with good outcomes after ECPR [54]. Several studies suggested that initial laboratory markers, including pH and lactate levels, can also identify favorable candidates for ECPR [34,55–57]. These laboratory markers are objective but not always available before ECPR implementation.
Several scoring systems have been proposed to assist in candidate selection for ECPR [58]. The TiPS65 score was developed using data from adult patients with shockable OHCA treated with ECPR in Japan. It is calculated by adding points from four variables: time from call to hospital arrival ≤25 minutes (Ti), pH ≥7.0 (P), shockable rhythm (S), and age <65 years (65). Validation studies of the TiPS65 score showed a C-statistic of 0.729 (95% confidence interval, 0.672–0.786) for prediction of 30-day survival with good neurologic outcomes [59]. The RESCUE-IHCA (Resuscitation Using ECPR During IHCA) score was developed using data from adult patients with IHCA treated with ECPR from Get With The Guidelines–Resuscitation (GWTG-R) [60]. This score considers six variables (age, preexisting renal insufficiency, time of day, illness category, presenting rhythm, and duration of cardiac arrest) and shows fair discriminatory (area under the curve, 0.676; 95% confidence interval, 0.606–0.746) and good calibration performances in a validation cohort from the ELSO registry. Clinical utility of these scoring systems remains to be determined.
Several studies have suggested that strict candidate selection criteria may improve ECPR outcomes [26,30,61–63]. A systematic review that assessed the effects of predefined selection criteria on survival after ECPR showed that an increased number of inclusion criteria was associated with improved outcomes in prospective studies [30]. However, using stricter criteria results in identifying fewer eligible patients, thereby increasing the risk of excluding potential candidates from receiving ECPR. Further studies are needed to determine candidate selection criteria that can maximize the benefits of ECPR while minimizing futile ECMO initiation.
Implementing ECPR
ECPR involves a series of time-critical interventions. Detailed information on the procedures, materials, and equipment necessary for ECPR (Fig. 2), should be included in ECPR protocols. Using preprimed ECMO circuits can reduce the time to ECMO initiation. Several studies reported that sterility of preprimed ECMO circuits remained uncompromised for more than 1 month [64,65]. Ultrasonography plays an important role in ECMO cannulation by localizing the femoral vessels and providing real-time imaging during cannulation (Fig. 3), leading to faster ECMO cannulation [66].

An example of a list of materials and equipment needed to perform extracorporeal cardiopulmonary resuscitation (ECPR). IV, intravenous; ECMO, extracorporeal membrane oxygenation.
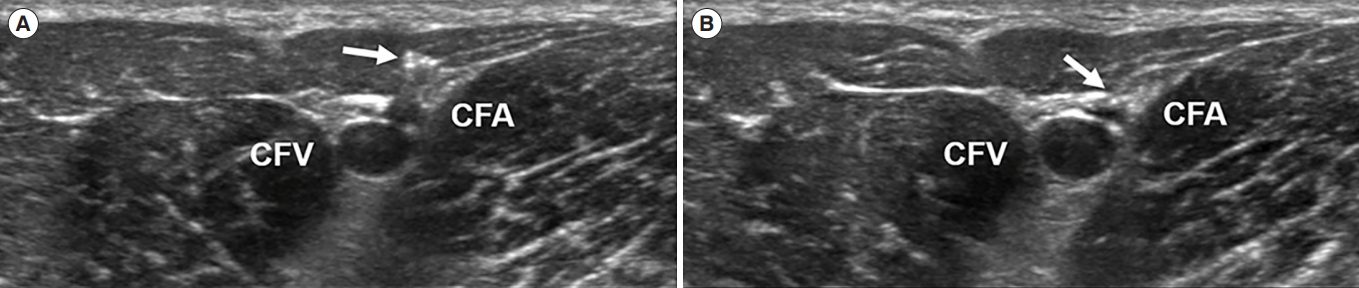
Transverse view of the common femoral artery and vein. Arrows point to shadow artifact of needle entering the femoral artery. (A) The needle tip is in the muscle layer above the common femoral artery (CFA). (B) The needle tip is about to enter the CFA. CFV, common femoral vein.
The percutaneous Seldinger or surgical cutdown techniques are the most commonly used for inserting the ECMO cannula into the common femoral artery and vein. Although the percutaneous Seldinger technique is more frequently used [29], it often fails even with ultrasonographic guidance [67]. The surgical technique is mostly used as a salvage measure after failed percutaneous access attempts and is associated with higher risk of local infection [68]. Which technique is better between the two remains unestablished, and the first choice of technique may depend on the operator’s preference and the patient’s anatomic characteristics. Chen et al. [67] reported that arterial diameter was significantly associated with the success of percutaneous ECMO cannulation using the Seldinger technique, with an arterial diameter of >4.5 mm yielding a relatively high success rate.
Because inadvertent venous placement of the arterial cannula can impede timely ECMO initiation, procedures to differentiate between arterial and venous puncture must be included in ECPR protocols. The color of blood taken at the puncture site can help in differentiating between arterial and venous puncture before guidewire insertion [69]. A guidewire-induced hyperechoic shadow within the inferior vena cava on ultrasonography confirms venous puncture (Fig. 4). Even after successful guidewire insertion, complications such as guidewire kinking or vessel perforation can occur during sequential dilations or cannula advancement. Moving the guidewire back and forth during sequential dilations and cannula advancement may help reduce complications. Upon sensing resistance to guidewire movement, advancement of the dilator or cannula should be stopped. When an arterial catheter is placed at the time of ECMO initiation, observing the arterial pressure waveform may also help in detecting cannula misplacement. Cannula misplacement should be suspected if there is no gradual increase in arterial pressure immediately after ECMO initiation (Fig. 5).

Ultrasonographic image showing guidewire-induced hyperechoic shadow (arrow) within the inferior vena cava.
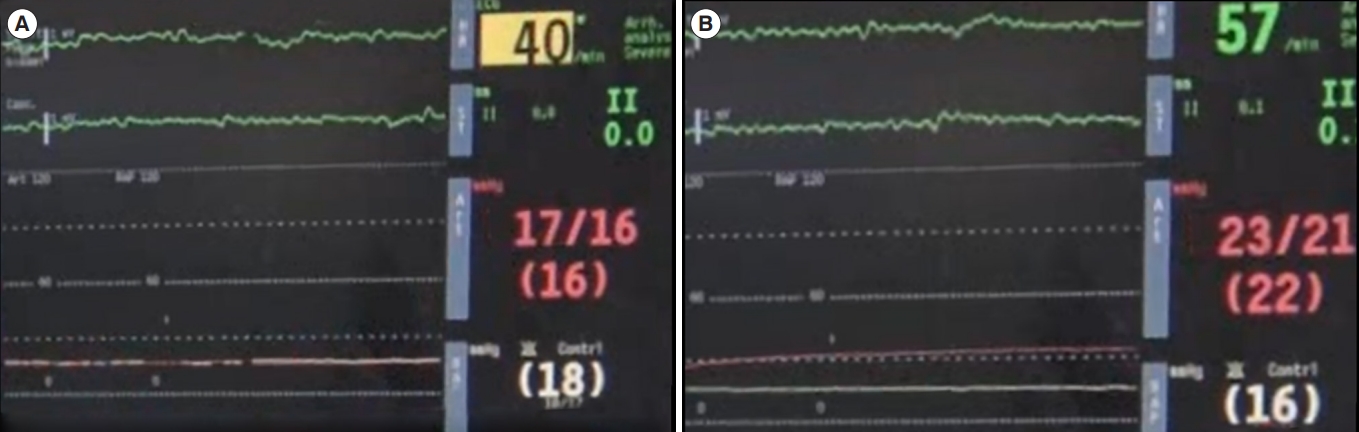
Screenshots of patient monitor (A) before and (B) immediately after extracorporeal membrane oxygenation (ECMO) initiation. Green, red, and white lines indicate electrocardiographic, arterial pressure, and right atrial pressure data, respectively. Note the progressive increase in the arterial pressure immediately after ECMO initiation.
ECMO should be initiated as soon as possible after the decision to apply ECPR is made. Placing angiocatheters in the femoral artery and vein during the initial assessment for ECPR candidacy and replacing the angiocatheters with ECMO cannulas after the decision to apply ECPR is made, rather than initiating ECMO cannulation after the decision, may help reduce the time taken to initiate ECMO [70]. High-quality CPR should be provided throughout the resuscitation until ECMO support is initiated. Multiple studies have reported no survival benefit of mechanical CPR over manual CPR [71,72]. However, the use of a mechanical CPR device may enable provisioning high-quality CPR until initiation of ECMO support and more space around the patient for ECMO cannulation, thus facilitating successful ECPR implementation.
Post-ECMO implementation care
Management after ECMO implementation should be part of ECPR protocols to ensure a highly protocolized sequence of care for patients, including diagnosis and treatment of arrest cause, hemodynamic and oxygenation support, monitoring and management of ECMO-related complications, and neuroprognostication. Several studies suggest a significant association between post-ECMO implementation care and good outcomes in patients undergoing ECPR [73,74].
Promptly diagnosing and treating the arrest cause after ECMO initiation can maximize chances of recovery. Patients with refractory cardiac arrest have a high prevalence of coronary artery disease (70%–80%) [75,76]. Therefore, emergent coronary angiography should be included in the ECPR protocol. Current ELSO guidelines recommend emergent coronary angiography for all patients undergoing ECPR without an obvious noncardiac etiology of arrest [53]. Multiple studies suggest a significant association between coronary angiography and/or percutaneous coronary intervention with improved outcomes in patients undergoing ECPR [36,73,77].
Current ELSO guidelines recommend routine computed tomography (CT) imaging of the brain, chest, and abdomen/pelvis as soon as possible in all ECPR cases [53]. Head CT helps in identifying intracranial hemorrhage, a common cause of cardiac arrest [78], and in predicting neurologic outcomes [78,79]. CT of the chest and abdomen/pelvis helps elucidate the cause of cardiac arrest, such as pulmonary embolism, and complications from prolonged CPR or ECMO cannulation. Several studies indicate the utility of whole-body CT after ECPR [80–82]. Osofsky et al. [82] evaluated the utility of whole-body CT in detecting clinically significant findings in 38 patients who underwent ECPR and whole-body CT. They reported that whole-body CT detected clinically significant findings in 37 patients (97%) and led to subsequent interventions in 20 patients (54%). ECMO poses unique technical challenges to contrast-enhanced CT imaging. For example, a significant volume of contrast material administered intravenously can be aspirated into the venous cannula and returned to the aorta via the arterial cannula, bypassing the pulmonary artery. ECMO flow needs to be temporarily reduced or stopped after contrast material administration to obtain images of sufficient quality to diagnose pulmonary embolism.
Treatment-refractory shock is the most common cause of death after ECPR [83]. Hemodynamic monitoring in patients receiving ECMO is challenging. Hemodynamic measurements using thermodilution techniques and those based on pulse contour analysis algorithms are unreliable during ECMO. An arterial line should be placed immediately (preferably in the right radial artery). Several studies reported significant associations between mean arterial pressure (MAP) and outcomes in patients resuscitated with ECPR [35,49,84,85]. In an observational study including 253 adult patients resuscitated with ECPR [49], patients with MAP of approximately 75 mmHg had the lowest probability of poor neurologic outcomes. Setting the MAP target to 60–80 mmHg according to the ELSO guidelines would be reasonable [53]. The optimal ECMO flow target in the early post-ECMO implementation period is unknown. The ECMO flow rate can be adjusted by referring to the method used in the ARREST trial [86]. In the ARREST trial, ECMO flow was maximized until vasopressors were discontinued and then decreased as tolerated to promote native cardiac function [86]. Pulse pressure on arterial pressure waveform is dependent on cardiac contractility and afterload and can be used to monitor hemodynamic state in patients undergoing venoarterial ECMO. A low pulse pressure has been associated with unsuccessful weaning from ECMO support and in-hospital mortality in patients undergoing ECPR [87,88]. Loss of pulse pressure indicates a predominance of blood flow through the ECMO circuit, with negligible blood flow through the native heart, which can cause left ventricular dilation. Left ventricular dilation can in turn cause pulmonary edema and myocardial injury, impeding cardiac recovery. Echocardiographic assessment of left ventricular dimensions and function and aortic valve opening help in diagnosing this complication. Several studies suggest a significant association between the use of mechanical left ventricular unloading and improved survival in patients undergoing ECPR [73,89]. ECPR protocols should include diagnostic and therapeutic options that can be used when pulse pressure is lost (e.g., inotropic support, afterload reduction, and intra-aortic balloon pump).
Upon ECMO initiation, sweep gas containing 100% oxygen is typically delivered at a flow rate matching the ECMO flow, frequently leading to hyperoxemia and hypocarbia. Pulmonary complications including pulmonary edema and acute respiratory distress syndrome frequently occur in patients with cardiac arrest [90,91]. Patients with impaired pulmonary gas exchange can be exposed to hypoxemia despite ECMO support because of a phenomenon known as Harlequin syndrome (difference in cerebral and lower extremity oxygenation due to hypoxic blood from the native heart flowing to the brain and hyperoxic blood from the ECMO circuit flowing to the lower extremities). Multiple studies reported significant associations between arterial blood gas derangement and poor outcomes in patients undergoing ECPR [73, 92–95]. In a retrospective study including 3,125 patients that received ECPR [92], severe hyperoxemia (≥300 mmHg) was associated with ischemic stroke, intracranial hemorrhage, and in-hospital mortality. Given the associations between arterial blood gas derangement and poor outcomes [92–96], frequent evaluation of arterial blood gases and careful titration of the ECMO gas blender setting are required to avoid arterial blood gas derangement that can adversely affect outcomes.
Whether targeted temperature management (TTM) improves outcomes of patients receiving ECPR remains unknown. Observational studies have yielded inconsistent results [36,97–99]. Randomized trials evaluating the effects of TTM on outcomes of patients treated with ECPR are lacking. However, given the high incidence of hypoxic-ischemic brain injury in patients undergoing ECPR [100], TTM can be reasonably considered for patients who remain comatose after ECPR. The ELSO guidelines recommend application of TTM targeting 33 to 36 °C for 24 hours to comatose patients after ECPR according to protocols that yielded excellent results [53,76,101].
ECMO is a highly invasive intervention with a high risk of complications [102,103]. Bleeding is a common complication and usually occurs at the cannulation site [104]. In the Prague OHCA study [21], bleeding was twice as common in the invasive strategy group (31%) than in the standard strategy group (15%). Cannulation site bleeding is usually controlled with manual pressure and rarely requires surgery [23,67]. Uncontrollable or serious bleeding within the central nervous system, thoracic cavity, or gastrointestinal tract, although uncommon, has been reported in patients undergoing ECPR [101,105]. In a study that investigated the effect of bleeding and red blood cell transfusion during ECMO on mortality [106], the volume of red blood cells transfused was significantly associated with in-hospital mortality. Screening measures for early detection of bleeding complications (e.g., complete blood count and ultrasonography) should be included in ECPR protocols.
Although anticoagulation strategies vary among ECMO protocols, anticoagulation is typically provided by an initial intravenous bolus of unfractionated heparin (5,000 units) followed by a continuous infusion titrated to maintain an activated clotting time (ACT) of 180 to 220 seconds or a partial thromboplastin time of 1.5 times the upper normal limit. Patients undergoing ECPR are prone to coagulation disorders, such as prolonged prothrombin time and partial thromboplastin time with thrombocytopenia [107,108], which may reduce anticoagulant requirements. Several studies suggest that avoiding the initial bolus dose or targeting low ACT levels may be helpful [109–111]. In a meta-analysis that evaluated the effect of different anticoagulant methods on bleeding/thromboembolic complications in patients receiving ECPR [111], the incidence of bleeding events and of thromboembolic events were higher among those who received an initial heparin loading dose than those who did not. In comparisons among the three different ACT level groups, the incidences of both bleeding and thromboembolic events were the highest in the high ACT group. Further studies are needed to confirm the safety and efficacy of the reduced anticoagulation strategy.
Limb ischemia distal to the arterial cannula site is a common complication of peripheral venoarterial ECMO, with an incidence of 10% to 20% [67,82,112]. A larger cannula size, diabetes, and CPR duration have been suggested as risk factors for distal limb ischemia [67,113]. Placing a distal limb perfusion cannula is recommended to prevent distal limb ischemia [53]. Distal limb perfusion cannula placement may not only decrease the risk of distal limb ischemia, but also improve survival in patients undergoing ECPR [73,114]. In a study including 7,488 adult patients treated with ECPR [73], placement of a distal limb perfusion cannula was independently associated with improved survival. Distal limb ischemia can still occur despite distal limb perfusion through the cannula [114]. Regular monitoring of distal limb perfusion should be included in ECPR protocols. Current ELSO guidelines suggest near-infrared spectroscopy for monitoring distal limb perfusion [53]. Several studies have suggested that monitoring calf tissue oxygen saturation using near-infrared spectroscopy may be useful for detecting distal limb ischemia [115,116].
According to current resuscitation guidelines [15], multimodal neuroprognostication should be performed at least 72 hours after achieving normothermia. Although whether the same approach reliably predicts neurologic outcomes in patients resuscitated with ECPR remains to be established, several studies have suggested that prognostic measures used for patients experiencing cardiac arrest not treated with ECMO may also be applicable to patients resuscitated with ECPR [117–119]. Ben-Hamouda et al. [117] compared the performance of prognostic measures, including pupillary reflex, electroencephalogram, somatosensory evoked potentials, and neuron specific enolase, between comatose cardiac arrest survivors treated with and without ECMO and reported comparable performances between both groups. Further studies are needed to identify the optimal neuroprognostication method in the ECPR population.
APPROACHES OF ECPR INITIATION FOR REFRACTORY OHCA
Three approaches are currently used for ECPR initiation in patients with refractory OHCA: initiation at an ECPR-capable hospital, prehospital initiation, and the rendezvous approach. The most commonly used approach is intra-arrest transport to an ECPR-capable hospital followed by initiation of ECPR at the hospital. The three published randomized trials on ECPR [20–22] followed this approach. Initiation of ECPR with this approach is the least complicated, because ECPR is typically performed by experienced providers at a high-volume ECMO center. However, with this approach, only a limited number of patients can receive ECPR within acceptable timeframes, because on-scene resuscitation and transport to a hospital usually take a significant amount of time. ECPR is only performed at a small number of tertiary hospitals, limiting the geographic coverage of ECPR. To overcome this limitation, alternative approaches including the prehospital initiation and rendezvous approach have been developed. In the rendezvous approach, an ECPR candidate is transferred to a hospital closer to the scene of arrest while an ECMO cannulation team is deployed to that hospital. The patient undergoes ECPR in the rendezvous hospital and is transferred to an ECMO center for postresuscitation care. The investigators of the ARREST trial extended their ECMO-facilitated resuscitation program to the Minneapolis–St. Paul metropolitan area using this approach and reported achieving a neurologically favorable survival rate similar to that in the ARREST trial (43%) [120]. Several systems, including the Service d’Aide Médicale Urgente of Paris, have adopted prehospital ECPR, which brings ECMO to patients with OHCA instead of taking the patients to an ECMO-capable center [26,121,122]. Lamhaut et al. [26] compared the mean CPR duration and survival rate before and after a change in the ECPR strategy in 156 patients treated with ECPR and reported a shorter mean CPR duration and higher survival rate in the prehospital ECPR-based strategy than in the strategy that allowed liberal allocation between prehospital or inhospital ECPR.
Several studies have suggested that the prehospital ECPR or rendezvous approach could allow more patients to receive ECPR than initiation on arrival to an ECPR-capable hospital [123,124]. Song et al. [124] quantified patient catchment areas of the three approaches in Sydney, Australia, and reported that the rendezvous (n=2,175,096) and prehospital ECPR models (n=3,851,727) substantially increased the catchment of eligible patients with OHCA compared to the in-hospital ECPR model (n=811,091). However, implementation of ECPR using these approaches is challenging. Both the rendezvous and prehospital ECPR approaches require substantial planning, training, and logistics efforts and highly coordinated collaboration between prehospital EMS and ECMO centers. The optimal approach for ECPR remains elusive, but may vary depending on geographical characteristics, population density, and medical resources of the region. The ECPR-capable hospital-based approach may be effective if multiple ECPR-capable hospitals are sufficiently dispersed across the region to handle most patients requiring ECPR. However, this is not the case in most regions, wherein the rendezvous approach or prehospital ECPR may be desirable to maximize the coverage of ECPR service.
CONSIDERATIONS FOR INCORPORATING ECPR WITHIN A SYSTEM OF CARE
All elements of the system of care for resuscitation of patients with cardiac arrest should be optimized to achieve neurologically favorable survival with ECPR. Efforts to increase provision of bystander CPR may improve outcomes of patients undergoing ECPR and increase the number of patients receiving ECPR [31]. Most patients with refractory OHCA are not eligible for ECPR due to a prolonged CPR time [125]. Efforts to reduce the time from cardiac arrest to ECMO initiation may therefore be critically important for successful ECPR implementation. Read et al. [126] reported a significant reduction in the time from cardiac arrest to ECMO initiation after implementing a dedicated simulation program for ECPR. Early communication and effective coordination between EMS and ECMO centers are also critical in limiting the time from cardiac arrest to ECMO initiation.
ECPR is only one part of the system of care for resuscitation of patients with cardiac arrest. Only a minority of patients with OHCA are ultimately considered suitable for ECPR [26,127,128]. Changes in EMS for successful ECPR implementation should not hinder resuscitation of patients not receiving ECPR. Intra-arrest transport for in-hospital ECPR can compromise CPR quality during transport, thus adversely affecting outcomes of patients not receiving ECPR [129,130]. Therefore, in cases of hospital-based ECPR, efforts should be made to select ECPR candidates requiring intra-arrest transport and to maintain high-quality CPR during intra-arrest transport (e.g., mechanical CPR).
ECPR requires a high level of expertise and experience. A recent study reported a significant association between higher ECPR case volume and improved survival [73]. ECPR-specific simulation training may help clinicians to develop and maintain the skills and experience needed to expeditiously and safely perform ECPR. Several studies have reported significantly reduced the time to ECMO initiation after ECPR-specific simulation training [126,131].
CONCLUSION
The efficacy of ECPR over conventional treatment in patients with refractory OHCA remains unclear. ECPR may render significant survival benefits over conventional CPR in selected patients only when performed with strict protocol adherence in an experienced system and with a high level of collaboration between EMS and ECMO centers. ECPR is only one part of the system of care for resuscitation of cardiac arrest victims. Optimizing the chain of survival is critical to improving outcomes of patients receiving ECPR.
Notes
ETHICS STATEMENTS
Not applicable.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: all authors; Visualization: YHJ; Writing–original draft: KWJ, YHJ; Writing–review & editing: all authors. All authors read and approved the final manuscript.
References
Article information Continued
Notes
Capsule Summary
What is already known
The use of extracorporeal cardiopulmonary resuscitation (ECPR) has significantly increased in recent years. Current resuscitation guidelines recommend its use as a rescue method in selected patients with refractory cardiac arrest.
What is new in the current study
Despite the increased use of ECPR, its clinical efficacy, suitable patient population, and optimal implementation strategy remain unclear. ECPR may have a significant survival benefit over conventional CPR in selected patients only when performed in an experienced system and strictly following protocol. Optimizing the chain of survival is critical to improving outcomes of patients receiving ECPR.