Development and demonstration of the protective efficacy of a convertible respiratory barrier enclosure: a simulation study
Article information
Abstract
Objective
The efficacy of previously developed respiratory barrier enclosures to limit healthcare workers’ exposure to aerosols from COVID-19 patients remains unclear; in addition, the design of these devices is unsuitable for transportation or other emergency procedures. Therefore, we developed a novel negative pressure respiratory isolator to improve protection from patient-generated aerosols and evaluated its protective effect in conversion to systemic isolator.
Methods
This in vitro study simulated droplets by nebulizing 1% glycerol + 99% ethanol solution. We performed cardiopulmonary resuscitation (CPR) and converted a respiratory barrier enclosure into a systemic isolator with a respiratory barrier as well as a respiratory barrier with negative pressure generator (NPG), which were compared with control and room air. During the procedure, particles were counted for 30 seconds and the count was repeated 10 times.
Results
During CPR, the total number of particles in the respiratory barrier with NPG (280,529; interquartile range [IQR], 205,263–359,195; P=0.970) was similar to that in the control (308,789; IQR, 175,056–473,276). Using NPG with a respiratory barrier reduced the number of particles to 27,524 (IQR, 26,703–28,905; P=0.001). Particle number during conversion of the respiratory barrier into a systemic isolator was also lower than in the control (25,845; IQR, 19,391–29,772; P=0.001).
Conclusion
The novel isolator was converted to a systemic isolator without air leakage. The aerosol-blocking effect of the isolator was quantified using a particle counter during CPR. Further studies comparing the barrier effect of isolators within various pressure differentials are warranted.
INTRODUCTION
Since the first reported cases of COVID-19, healthcare workers (HCWs) have faced new challenges in resuscitating patients with COVID-19 or those who are at risk but not infected [1]. Approximately 5% to 10% of HCWs who treated patients with COVID-19 have reported being infected with COVID-19 despite the use of personal protective equipment (PPE) [2]. Infected HCWs are at risk of spreading the infection to other vulnerable individuals, may suffer threats to their own health, and may as a consequence exacerbate their own shortage during severe outbreaks [3,4]. Although the use of PPE is recommended to prevent the spread of COVID-19, demand for it outweighs supply, thus significantly limiting HCW protection [5]. The shortage of PPE and risk of COVID-19 infection in HCWs mandate the need for alternative methods of protection. The plastic shield box, also known as barrier enclosure, aerosol box, or intubation box, is an effective protective device that has been developed for a variety of situations [6,7]. These newly developed devices are designed to protect HCWs and have been shown to be at least partially protective during aerosol-generating procedures, such as endotracheal intubation, extubation, and cardiopulmonary resuscitation (CPR) [6,8]. However, evidence regarding their efficacy in preventing infection and efficiency in various healthcare situations is limited [6,8,9].
During the COVID-19 pandemic, HCW protection is important not only during aerosol-generating medical procedures but also during the transportation of suspected or diagnosed patients for definitive care [10–12]. In addition, reducing the chances of contamination during other emergency procedures, such as coronary angiography, intra-arterial thrombolysis, and bleeding control through gastroscopy in infected patients is important. These considerations for the protection and isolation of HCWs delay definitive emergency treatment of acute myocardial infarction, acute stroke, and abdominal surgery in patients suspected to have COVID-19 [13–15]. However, current barrier enclosures are unsuitable for patient transport and even for performing non–aerosol-generating procedures, such as coronary angiography, intra-arterial thrombolysis of stroke patients, and abdominal surgery [7].
In this study, our board-certified emergency physician– and mechanical engineer–based team aimed to develop a novel negative pressure isolator that is adaptable in various situations and easily convertible to a systemic isolator. We subsequently tested its protective efficacy.
METHODS
Intubation hood with patient access orifices
Our team designed a respiratory isolation hood for intubation and other aerosol-generating procedures using a three-dimensional (3D) design program (Rhinoceros 3D ver. 7.0, McNeel). The height of the patient access orifice was determined according to the physical build of the HCW performing endotracheal intubation. Current aerosol boxes are rectangular [6]; hence, the target location of intubation is difficult to visualize. We considered this limitation and installed a slope from the patient access orifice to the distal portion of the hood accordingly.
Tailored double-layer barrier for chest enclosure
The chest barrier was designed to enclose the patient’s breathing field with the following considerations. First, it was double layered to provide an anteroom structure as in the current negative pressure isolation rooms. Second, rubber bands and drawstrings were used to establish different isolation ranges according to the patient’s body size and condition. Finally, the outer layer of the barrier had a Velcro attachment with a polyvinyl chloride (PVC) half-isolator that converted it into a systemic isolator, such as in a conventional cart-type systemic isolator.
Negative pressure generator
In our preliminary study [16], we found that fan-type ventilation machines produced rapid airflow and efficiently contained aerosols with low-pressure differentials compared to room air. We used a previously constructed fan-type ventilation machine that could generate –10 Pa of pressure differential relative to room air and measured such pressure using a sensor (Differential Pressure Transmitter 984, Beck Sensortechnik GmbH). The ventilation machine was called the “negative pressure generator (NPG).” We connected this NPG to the isolator via an 80-mm diameter tube.
Aerosol and droplet simulation
To simulate aerosol and droplets from patients, we used vapor and nebulized droplets. To visualize air flow, an Antari fog machine Model Z-800II (Antari) was used to release buoyant water vapor inside the hood for 10 to 30 seconds. To create droplets, we nebulized 5 mL of a 1% glycerin in 99% ethanol solution next to the mannequin's mouth using an Omron Compressor Nebulizer Model NE-C802 (Omron Healthcare Korea Co Ltd).
Observation and quantification of air leakage during CPR
Air leakage from the isolator was visually observed and quantitatively assessed during CPR. To compare leakage during CPR, the isolator was first filled with vapor, and then a researcher performed CPR under NPG on/off conditions without a chest barrier, with an inner barrier, and with a double-layer barrier. We visually inspected vapor leakage under these six conditions.
To quantify droplet leakage from the isolator, an AeroTrak Portable Particle Counter 9306 (TSI Inc) was positioned next to the CPR operator's head (80 cm above the bed). This particle counter used a laser diode and a photo detector to count airborne particles within the size ranges of 0.3, 0.5, 1.0, 3.0, and 5.0 µm. The particle counter was set up to analyze droplets by aspirating 2.8 L of air every 30 seconds. One researcher performed chest compressions for 30 seconds without a chamber (control), with a chamber (chamber condition), and with chamber-operating NPG (NPG condition) and the experiment was repeated 10 times for each condition (Supplementary Fig. 1).
Observation and quantification of air leakage during conversion
Aerosol and droplet containment during isolator conversion was also examined. Two researchers converted the respiratory barrier enclosure into a systemic isolator while operating NPG, while another researcher utilized the particle counter at the same position as that used during the CPR.
Statistical analysis
In the pilot test, the difference between baseline of particles in regular room air and those in the air after nebulizing for 2 minutes was calculated, and the mean difference of particles was 268,168±204,952. The sample size for the detection of 1% mean difference in 2,682±2,050 particles with a two-sided significance level of 5% and power of 95% was 7; therefore, particle count was performed 10 times for each condition. To test the normality in each condition, Shapiro-Wilk test was conducted, and the difference between the groups was analyzed using an independent t-test when normality was satisfied, and Mann-Whitney U-test when normality was not satisfied. All statistical analyses were performed using SAS ver. 9.4 (SAS Inc). A P-value of <0.05 was considered statistically significant.
RESULTS
Intubation hood and tailored double-layer barrier for the chest
We fabricated a mock-up of the hood (Fig. 1) and the double-layer barrier structure (Fig. 2) to test the newly designed device. The height of the hood was approximately 500 mm and that of the patient access orifices was 380 mm. This difference in height formed an inclined plane in the visual field of the operator. This design facilitated the procedure by providing the operator a closer view of the patient’s larynx. The intubation hood had two other patient access orifices for the assistant or various connectors for oxygen or medications.

The three-dimensional (3D) design of (A) the intubation hood and (B) the mock-up. (A) The Rhinoceros 3D ver. 7.0 (McNeel) is used to design the intubation hood with the appropriate height and width for intubation and computed tomography scans. (B) The hood mold was built using a transparent acrylic resin.

Double-layer barrier made of a waterproof fabric on the side of the patient. (A) The inner layer of the double-layer barrier is secured to the patient’s chest using a rubber band and a Velcro fixer. (B) Changeable outer layer of the double-layer barrier secured using a drawstring and stopper. (C) Converted systemic isolator. The outer layer is equipped with a Velcro attachment (arrow) with the polyvinyl chloride half-isolator to convert it into a full body isolator.
The double-layer barrier was made of a coated waterproof fabric. The inner layer could be secured to the patient’s chest using a rubber (latex-free) band and the Velcro fixer (Fig. 2A), and the changeable outer layer could be secured anywhere between the patient’s chest and waist using the drawstring (Fig. 2B). The space between the two barriers prevented internal air leakage during chest compressions or postural changes and maintained the internal negative pressure.
The outer layer also had a Velcro attachment with the PVC half-isolator for converting the respiratory barrier enclosure to a systemic isolator. The presence of the inner layer during this conversion prevented air leakage and maintained negative pressure in the intubation hood (Fig. 2C).
Observation and quantification of air leakage during CPR
The generated vapor filled the isolator in 10 seconds. Without the double-layer barrier, most of the vapor escaped from the intubation hood within the first 30 seconds of the test. After the inclusion of the inner layer, the vapor in the intubation hood escaped to a lesser extent. However, during chest compressions, considerable vapor leakage was evident from the patient-layer connection (Fig. 3A). After the inclusion of the outer layer, vapor leakage was diminished but still obvious (Fig. 3B). When the NPG was operated to achieve an isolator pressure of approximately 10 Pa less than room air pressure, a small amount of vapor leaked from the inner layer (Fig. 3C), but no leakage was evident from the outer layer even after performing chest compressions (Fig. 3D and Supplementary Video 1). No visible vapor leakage was evident when the respiratory barrier enclosure was converted to a full body isolator while operating the NPG (Figure 4 and Supplementary Video 2).

Vapor leakage during chest compressions. (A) Inclusion of the inner layer and chest compression procedure. There is considerable vapor leakage (arrows) at the patient-inner layer interface. (B) Inclusion of the outer layer decreases the amount of vapor leakage, but it continues to persist. (C) Inclusion of the inner layer with negative pressure decreases the amount of vapor leakage (arrow) compared with that in (A). (D) After the inclusion of the outer layer with negative pressure, there is no vapor leakage visible even after performing chest compressions.

Respiratory barrier enclosure converted into a systemic isolator. (A) Velcro connection (arrow) between the base of the outer layer and polyvinyl chloride (PVC) half-isolator. (B) Velcro attachment at each sidewall (arrow). (C) Closing the midline zipper. (D) Systemic isolator (arrow, Velcro attachment site).
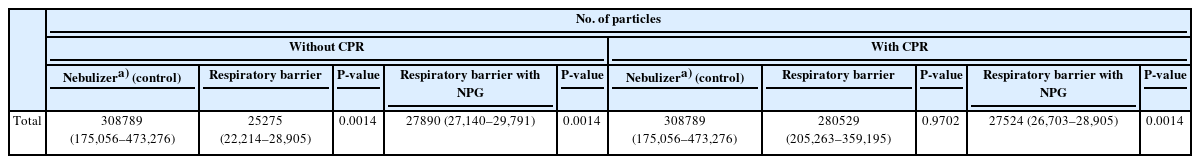
The nebulizer generated 200,000 to 300,000 particles. To quantitatively assess the efficacy of droplet containment by the isolator, we conducted nebulization without the isolator and measured the airborne particle count. This served as a comparative baseline and was labeled as the nebulizer (control). The total number of particles in the control group (308,789; interquartile range [IQR], 175,056–473,276; P=0.001) was significantly higher than that in room air (19,664; IQR, 18,088–21,562) (Supplementary Table 1). Applying a respiratory barrier enclosure with a double-layer barrier significantly reduced the total number of particles in the air by 25,275 (IQR 22,214–8,905; P=0.001). However, during chest compressions, the total number of particles in the air was not significantly decreased (280,529; IQR, 205,263–359,195; P=0.970), in contrast to when the NPG was in use (27,524; IQR, 26,703–28,905; P=0.001) (Table 1). This result shows that a respiratory barrier enclosure with negative pressure (–10 Pa) could reduce aerosol and droplet transfer from the patient to the CPR performer.
Comparison of the number of particles around the airway of the CPR operator with the number of particles in the control
However, lower particle count measured compared to the control does not imply complete containment. If the isolator completely blocked particle escape, there should have been no increase in particle quantity when compared to the baseline particle count of room air with the nebulizer placed inside. When compared with the baseline particle level in room air (baseline), the total number of particles in the air increased even when using the respiratory barrier enclosure with NPG during CPR (27,722±1,239 vs. 21,125±3,245, P=0.001) (Table 2).
Observation and quantification of air leakage during conversion
Most of the vapor inside the respiratory barrier enclosure was removed within 15 seconds using an NPG. We also attempted to measure the air-cleaning time in the systemic isolator, since the presence of the inner layer caused some difficulties in transferring the generated vapor from the intubation hood to the PVC half-isolator. After the vapor was generated, most of it was removed from the systemic isolator in 120 seconds (Supplementary Video 3).
The total quantity of particles counted after the conversion of the respiratory barrier enclosure to the systemic isolator (conversion group) was significantly lower than the quantity in the control group (25,845; IQR, 19,391–29,772; P=0.001) (Table 3).
DISCUSSION
This in vitro simulation study reported the development and assessment of a convertible respiratory barrier enclosure with an intubation hood and a double-layer barrier. This isolator should protect HCWs from respiratory infection during airway and non-airway management as well as during patient transport. The device was effective in blocking aerosols and droplets from the patient’s respiratory tract, and this protective effect was maintained during chest compressions, and during conversion to a systemic isolator. Once this device is correctly applied to a patient, it can be used for transportation and other procedures, enabling the performance of emergency procedures even in an environment without isolation facilities.
This novel isolator has properties that allow it to overcome several disadvantages of previous barrier enclosures. It has four large patient access orifices for the full range of motion of the operator’s arm and it has separate spaces to prevent aerosol leaks during procedures. Our device is more comfortable to use than the aerosol box and is associated with a lower risk of destroying PPE during operation [17,18]. Additionally, the opening-type patient access orifices offer improved patient access compared to those used in the enclosure type [8,19]
The double-layer barrier is associated with a lower risk of air leakage. We found that a single layer barrier could not contain vapor with a low-pressure difference. This demonstrates the need for a double-layer barrier (Fig. 3). In addition, most current barrier enclosures were designed with a head cover and patient access orifices in mind, but with no consideration for enclosing other body areas [8]. Consequently, a protective effect for the operator performing intubation was evident, while no protective effect for the patient was observed [7]. Therefore, the use of such barrier enclosures is limited to during intubation and other aerosol-generating procedures. However, in this study, our novel double-layer barrier had a protective effect on the patient body side. Hence, it is feasible to use this device during other procedures and to utilize it instead of an isolation room. Our novel isolator can also be converted into a conventional negative pressure cart for patient transportation and vice versa without aerosol leakage. This conversion can provide increased protection for HCWs during patient transport for emergency procedures or for in-hospital transport. In addition, a variety of invasive procedures such as endoscopy and angiography could be performed without getting on and off the converter.
This novel isolator uses a high-flow fan-type air circulator to create a negative pressure environment in the enclosure. It differs from other enclosures using wall suction or other types of vacuum pumps [6–8,19]. In addition, the pressure difference between room air and the isolator was verified considering patient safety and the effect of air suctioning on the patient. Furthermore, we also observed that a pressure difference of approximately 10 Pa was sufficient to contain the aerosol and to quickly remove the particles in the air from inside the isolator.
This isolator has potential limitations. First, its intubation hood is relatively heavy, making it difficult for one HCW to secure the device to the patient’s head. Hence, this could lead to accidents during the quarantine period. However, our test hood was a mock-up model, and the thickness of the hood was not considered. A heavy hood can be addressed by fabricating a lighter hood with thinner walls. Second, we did not test for air leakage from the air circulator. Our team used a high-efficiency particulate air filter with a 0.3-µm filter and 99.7% efficacy, under the assumption that it would not leak. Depending on the type of filter or method of application, the infected air can contaminate the room. Third, the size of our device may be inadequate for patients with obesity and those with broad shoulders. However, our intubation hood is approximately 500 mm in width and height, which is suitable for bed size, and can even be fitted into a computed tomography machine. Fourth, although we clarified that the pressure difference of approximately 10 Pa was sufficient to reduce air contamination, there is limited evidence about proper pressure differential for the containment of aerosol and droplets without harmful effects on patients. Further studies must be conducted to compare the barrier effects of isolators within various pressure differential conditions and to clarify optimal pressure differential. Fifth, although the double-layer barrier with negative pressure showed high efficacy in aerosol containment, a small amount of particle leakage was detected during CPR, compared to the baseline room air. This indicates that there is room for improvement in the protective efficacy, and further studies are needed to enhance containment measures. However, when comparing each group based on particle size, it was observed that the smaller the particle, the more leakage occurred. When operating NPG, whether during CPR or conversion, the number of particles larger than 3 µm was not significantly different from that in the baseline room air (during CPR: 3 µm, P=0.079; 5 µm, P=0.336; 10 µm, P=0.214; during conversion: 3 µm, P=0.724; 5 µm, P=0.255; 10 µm, P=0.718) (Supplementary Table 2). Considering that the average size of patient droplets is about 2 to 3 µm, the observed containment of particles larger than 3 µm is sufficient to protect HCWs from airborne diseases. Sixth, this study focuses on the development of a convertible isolator, capable of transforming from a whole-body isolator to a hood-type barrier. This implies that the containment efficacy of this novel isolator must be compared not only with hood-type isolators but with other transportation chambers as well. However, there is currently no available published data about transportation isolators, except for pressure information and filter efficacy. As a result, we were not able to directly compare the containment efficacy of our novel isolator with these types of isolators. However, the new development type isolator may have comparable containment efficacy, along with a high-efficiency particulate air (HEPA) filter (99.7%), which could negate the need for comparison with other chamber's efficacy. Further study is needed to accurately and quantitatively measure the containment efficacy itself. Finally, this study is only a simulation; thus, further studies are needed to confirm its applicability on real patients. In addition, considerations on the efficiency of performing procedures with the hood are needed. Therefore, simulation studies addressing the efficiency and limitations when performing procedures with this novel isolator must be conducted in the future. Aerosol dispersal during application and removal of the chamber, similar to donning or doffing PPE, especially needs to be investigated.
In summary, we developed a novel negative pressure isolator that included an intubation hood with patient access orifices, a double-layer barrier, and an efficient NPG with an air circulator and pressure detector. This novel isolator can be converted to a systemic isolator without air leakage. We quantified the aerosol-blocking effect of the isolator using a particle counter during aerosol-generating procedures. Future studies addressing the efficiency and limitations when performing procedures using our novel isolator and comparing the barrier effect of isolators within various pressure differential are warranted. We anticipate that this novel isolator can be used for the treatment of infected patients without the risk of infection. It is expected to be highly beneficial in coping with potential hazardous infectious diseases following COVID-19.
Supplementary materials
Supplementary materials are available from https://doi.org/10.15441/ceem.23.067.
Supplementary Table 1.
Count of particles in room air with or without droplet generation
Supplementary Table 2.
Comparison of large-sized particles around the airway of the CPR operator and that of room air
Supplementary Fig. 1.
Researcher's position during cardiopulmonary resuscitation (CPR) (A) without an isolator and (B) with an isolator.
Supplemental Video 1.
Air leakage during chest compression. (A) Inner layer with negative pressure. (B) Double layer without negative pressure. (C) Double layer with negative pressure. Application of negative pressure in the double-layer barrier stops vapor leakage during chest compressions.
Supplemental Video 2.
The entire sequence of conversion from the respiratory barrier enclosure to a systemic isolator. The total procedural time is 70 to 90 seconds. No vapor leakage is evident during conversion with the negative pressure generator.
Supplemental Video 3.
Air clearance from (A) the respiratory barrier enclosure and (B) the systemic isolator.
Notes
Author contributions
Conceptualization: JYH, KSS; Data curation: MHP; Formal analysis: JYH; Funding acquisition: JYH; Investigation: MHP, JWM; Methodology: JHK, MHP, JYH; Project administration: KSS, JYH; Resources: KSS; Supervision: JYH; Validation: KSS; Visualization: KSS; Writing–original draft: MHP, JYH; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Conflicts of interest
The methods used in this study is currently under patent pending in Korea (No. 10-2021-0072895), and the authors may receive remuneration if the technology is transferred. However, it has not yet been commercialized, the authors have not received any financial benefit from this study. The authors have no other conflicts of interest to declare.
Funding
This study was supported by a Korea Health Technology R&D Project grant (No. HG22C0001) through the Korea Health Industry Development Institute (KHIDI), funded by the Korean Ministry of Health and Welfare.
Acknowledgements
The authors thank Crenvitec Co Ltd (Gwangju, Korea) for granting access to the use of the particle counter.
Data availability
Data analyzed in this study are available from the corresponding author upon reasonable request.
References
Article information Continued
Notes
Capsule Summary
What is already known
Healthcare worker protection is important during the transport of suspected or diagnosed respiratory patients for definitive care and during aerosol-generating medical procedures; this became especially apparent during the COVID-19 pandemic. However, the barrier enclosures currently in use offer inadequate protection to healthcare workers during patient transport and even during non–aerosol-generating procedures, such as coronary angiography, intra-arterial thrombolysis of stroke patients, and abdominal surgery.
What is new in the current study
In this study, our board-certified emergency physician– and mechanical engineer–based team aimed to develop an intubation hood with patient access orifices that could be closed and opened using an iris diaphragm, and a negative pressure generator with a pressure differential detector. We successfully developed a novel negative pressure isolator which could be transformed into a systemic isolator without air leakage when we quantified using a particle counter during cardiopulmonary resuscitation.


