Treatment of acute carbon monoxide poisoning with induced hypothermia
Article information
Abstract
Objective
The effect of induced hypothermia on severe acute carbon monoxide (CO) poisoning remains to be addressed further. We investigated the effect of induced hypothermia on severe acute CO poisoning.
Methods
Retrospective chart review was conducted for patients who diagnosed as severe acute CO poisoning in emergency department and underwent induced hypothermia from May 2013 to May 2014. Hospital courses with critical medication and major laboratory results were investigated through the chart review.
Results
Among total 227 patients with acute CO poisoning during the period of study, patients with severe acute CO poisoning were 15. All patients underwent induced hypothermia with a temperature goal 33°C. Initial and follow-up levels of S100B protein after induced hypothermia were 0.47 μg/L (interquartile range, 0.11 to 0.71) and 0.10 μg/L (interquartile range, 0.06 to 0.37), respectively (P = 0.01). The mean Glasgow Coma Scales at emergency department admission was 6.87 ± 3.36. Except 1 patient who expired after cardiopulmonary resuscitation, Glasgow Coma Scales at 30-day of hospital discharge were 15 in 10 patients (71.4%), 14 in 1 patient (7.1%), 13 in 1 patient (7.1%), and 6 in 2 patients (14.2%). Seven patients (46.7%) developed delayed neurologic sequelae. Four patients showed mild types of delayed neurologic sequelae and 3 showed moderate to severe types of delayed neurologic sequelae.
Conclusion
Most of patients underwent induced hypothermia had a good recovery from severe acute CO poisoning. Therefore, induced hypothermia may be considered as a possible treatment in severe acute CO poisoning.
INTRODUCTION
Acute carbon monoxide (CO) poisoning has one of the highest poisoning mortality rates worldwide [1]. The incidence rate of acute CO poisoning rapidly increased in South Korea as burning ignited charcoal recently gained use as a suicide method [2]. Although treatments remain controversial, hyperbaric oxygen therapy (HBOT) is the most recommended treatment for severe acute CO poisoning [3,4]. Previous reports demonstrated that HBOT decreased delayed neurologic sequelae (DNS) incidence [3,4]. However, other reports have shown that HBOT was not effective in DNS prevention [5,6]. Even if HBOT was not so effective, excepting HBOT, there were no clearly recommended methods in the treatments of severe acute CO poisoning.
In induced hypothermia, the body’s core temperature is maintained between 32°C and 34°C during an early period of treatment to provide a neuroprotective effect in patients with suspected brain injury [7,8]. Direct tissue hypoxia, systemic inflammatory responses including apoptosis secondary to tissue hypoxia, and reperfusion are the major injury mechanism in acute CO poisoning. The heart and brain are major target organs injured in acute CO poisoning. Considering these facts, induced hypothermia is expected to play a major role in treating moderate to severe acute CO poisoning. Although anecdotal case reports have shown a good prognosis after induced hypothermia in patients with severe acute CO poisoning, the current clinical indications of induced hypothermia have not included acute CO poisoning [9,10]. The effect of induced hypothermia on severe acute CO poisoning remains to be addressed further. Therefore, we investigated the effect of induced hypothermia on severe acute CO poisoning.
METHODS
This is a retrospective case series study. Patients diagnosed with severe acute CO poisoning during the 13 months from May 2013 to May 2014 were selected. This study was approved by a local institutional review board. The criteria for severe acute CO poisoning were history of CO exposure and any of Glasgow Coma Scale score <9 after acute CO exposure, blood carboxyhemoglobin level >25%, and S100B protein level >0.165 µg/L [11]. A monochamber (Nambuk Inc., Suwon, Korea) was used for HBOT. Indications for HBOT were any of the following: CO hemoglobin level >25%, depressed mental status, pregnancy, dysrhythmia after CO exposure, focal neurologic changes, persistent hypotension, and persistent acidosis [11]. However, patients who could not breathe spontaneously, those who needed continuous monitoring of vital signs, and those who were intubated were excluded from HBOT. Within 48 hours of emergency department admittance, when able to receive HBOT, the patients received 2 HBOT treatments to a maximum pressure of 2.6 to 2.8 atmosphere absolute for 90 minutes of O2 breathing time. Induced hypothermia was applied to all patients to protect against brain injury. Induced hypothermia was carried out by surface cooling using Arctic Sun 2000 (Medivance, Louisville, CO, USA). Arctic Sun pads cover the chest wall and both thighs of patients and are used to maintain a body core temperature between 32°C and 34°C. Sedatives (dexmedetomidine and remifentanil) and paralytics (cisatracurium or vecuronium) were administered to the patient to rapidly decrease body temperature and prevent shivering. Water mist was simultaneously sprayed on the whole body except the areas covered by the Arctic Sun to rapidly decrease body temperature. DNS were defined as continuous alterations in mental status, Parkinsonism features, psychosis, cognitive dysfunction, memory impairment, difficulty communicating with others, difficulty walking, or urinary incontinence at hospital discharge. The patient’s medical records were reviewed. Demographic, clinical, laboratory, and radiologic data were extracted by 2 trained emergency physicians. Laboratory studies including levels of S100B protein, catecholamines (epinephrine, norepinephrine, and dopamine), CO hemoglobin, and lactic acid were performed when the patient visited the emergency department. Follow-up S100B protein levels were checked within 86 hours of induced hypothermia.
We described our continuous data by their mean and standard deviation or median (interquartile range). Sign test was used to compare initial and follow-up S100B protein levels. Statistical significance was determined as P<0.05.
RESULTS
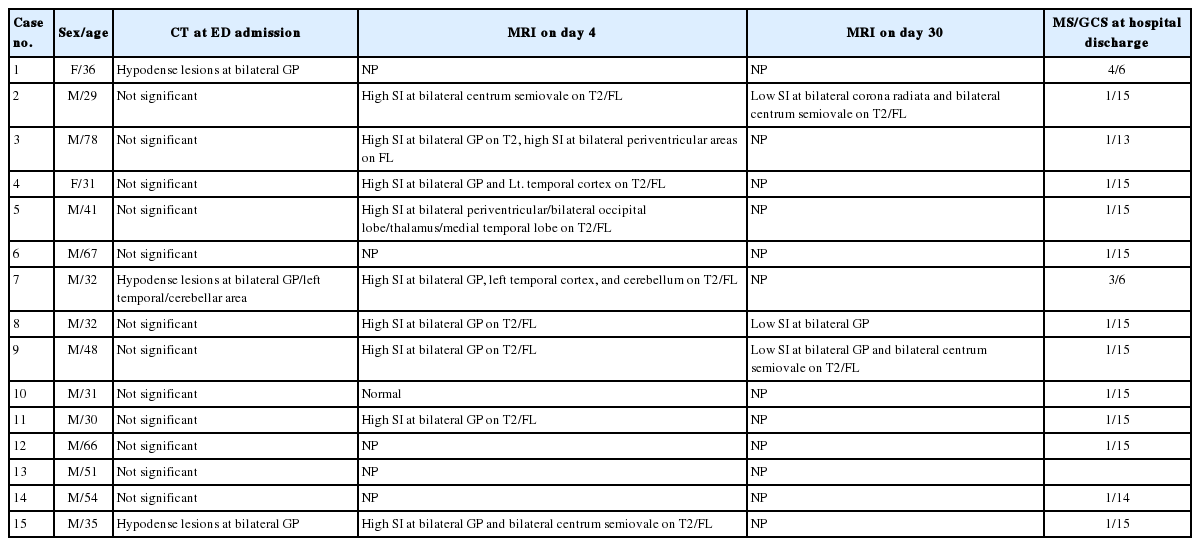
A total of 15 patients with severe CO poisoning were selected to participate in the present study. The mean age and sex ratio were 44.1±15.9 years and 1:0.8, respectively. The radiologic findings, Glasgow Coma Scale score, and mental status at hospital discharge are shown in Table 1. Seven patients (46.7%) demonstrated DNS at discharge and 1 (6.5%) who had undergone cardiopulmonary resuscitation expired. Of the 7 patients with DNS, 2 had severe DNS (continuous depression in mental staus), and the others showed mild to moderate DNS (bradykinesia, decreased responses to stimuli, cognitive dysfunction, memory loss, and difficulty memorizing).
The mean CO level was 22.6±17.0% at admission, ranging from 1.2% to 50.2%, since several patients had been transferred to our emergency department from other hospitals after high O2 oxygenation. The S100B protein levels at admission and follow-up were 0.47 µg/L (interquartile range [IQR], 0.11 to 0.71) and 0.10 µg/L (IQR, 0.06 to 0.37), respectively. After induced hypothermia, follow-up S100B protein levels significantly decreased (P=0.01). Considering catecholamine levels, the median epinephrine level was 0.12 ng/mL (IQR, 0.11 to 0.71; reference, 0.00 to 0.30 ng/mL), the median norepinephrine level was 0.85 ng/mL (IQR, 0.33 to 2.42; reference, 0.00 to 0.80 ng/mL), and the median dopamine level was 0.12 ng/mL (IQR, 0.02 to 0.26; reference, 0.00 to 0.20 ng/mL). The other laboratory results and the result of radiologic findings are shown in Tables 1 and 2, respectively.
Possible complications after induced hypothermia were related to infections. Six patients had pneumonia, and 1 had acute respiratory distress syndrome. Although pneumonia developed in these 6 patients, all patients recovered from pneumonia and acute respiratory distress syndrome.
DISCUSSION
Until now, although HBOT was recommended as the best treatment for patients with severe acute CO poisoning, its effectiveness in preventing DNS remains controversial. Despite encouraging experimental and clinical data obtained so far that may suggest a potential future indication in patients with acute CO poisoning, routine use of induced hypothermia in patients with acute CO poisoning requires further investigation [12,13]. We found that induced hypothermia significantly decreased S100B protein follow-up levels (within 96 hours) in patients with severe CO poisoning, and despite our series only including patients with severe acute CO poisoning, the DNS occurrence rate was similar to that found in a previous study [11]. Although catecholamines (epinephrine, norepinephrine, and dopamine) were not drawn from the synapses of the nervous system or cerebrospinal fluid, their levels were increased in the patients in this study (Table 1). We recently proposed that DNS was likely the result of delayed and ongoing inflammation associated with catecholamine or serotonin in the brain [14]. We speculate that, aside from ischemic hypoxia, reperfusion injury including nitrogen response to ischemia, immune responses related to reperfusion injury and catecholamine surge play important roles in the pathogenesis of acute injury and DNS after CO exposure. However, the clear mechanism of DNS after acute CO poisoning remains to be elucidated in further studies.
Arrich et al. [7] demonstrated that the neuroprotective effects of induced hypothermia resulted from inhibition of the biosynthesis, release, and uptake of catecholamines and of glutamate and dopamine neurotransmitters involved in the generation of the free radicals and cellular mediators responsible for the brain injury. These results have great implications for the treatment of acute CO poisoning. We think that induced hypothermia will be a good treatment for severe acute CO poisoning, because it has been known as the best way to minimize systemic inflammation. We also infer that induced hypothermia suppresses further catecholamine excess, reactive oxygen species generation, and subsequent lipid peroxidation, thereby preventing DNS from developing after acute CO poisoning.
As shown in Table 3 [9,10,15], previous studies regarding induced hypothermia in acute CO poisoning, except for that by Feldman et al. [15], showed that hypothermia maintenance time was relatively long, ranging from 3 to 7 days. The induced hypothermia maintenance time in our series also ranged from 32 to 67 hours, which was longer than that of induced hypothermia for current standard indications (ranging from 12 to 24 hours). This is based on the speculation that the dissociation time of the CO complex bound with hemeproteins including hemoglobin, myoglobin, and others to free CO is longer than 24 hours, and, in the context of hypothermia, dissociation time is more delayed. Extended induced hypothermia leads to infection, which is the most catastrophic complication [11]. Although pneumonia developed in 6 patients, the risks of infection and bleeding secondary to extended induced hypothermia were not problematic in our series. Therefore, we believe that the duration of induced hypothermia may be extended in acute CO poisoning unless there are suspicious signs of infection and bleeding. Further study will be needed to clarify this speculation.
In conclusion, induced hypothermia significantly decreased S100B levels and resulted in good recovery of the patients in our study. Although acute CO poisoning is not yet a standard indication for induced hypothermia, in cases when HBOT is limited owing to unstable vital signs and intubation or absence of an available HBOT, induced hypothermia may be considered the main treatment and play an important role in treating patients with acute CO poisoning.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Although hyperbaric oxygen therapy is currently a well known treatment method, its usefulness for delayed neurologic sequelae is controversial. Although several anecdotal cases showed good prognosis after induced hypothermia in the treatment of patients with severe acute carbon monoxide (CO) poisoning, the effect of induced hypothermia on acute CO poisoning remains to be addressed.
What is new in the current study
Most patients who underwent induced hypothermia showed good recovery from severe acute CO poisoning. Therefore, induced hypothermia may be considered as a possible treatment in severe acute CO poisoning.