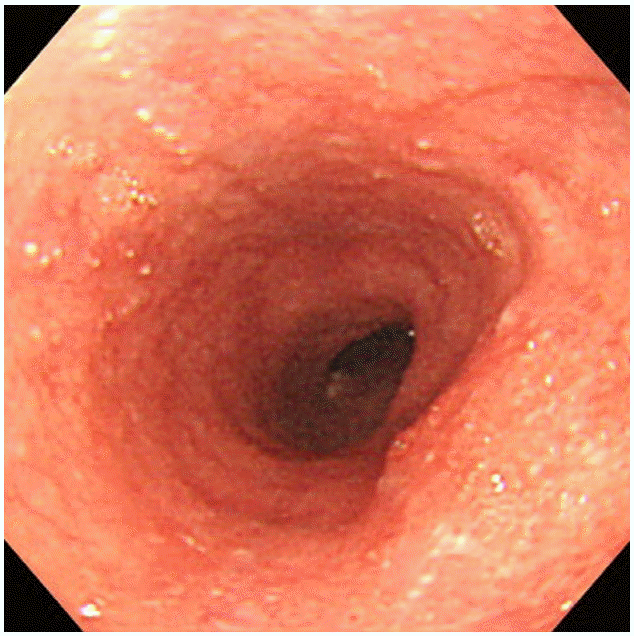
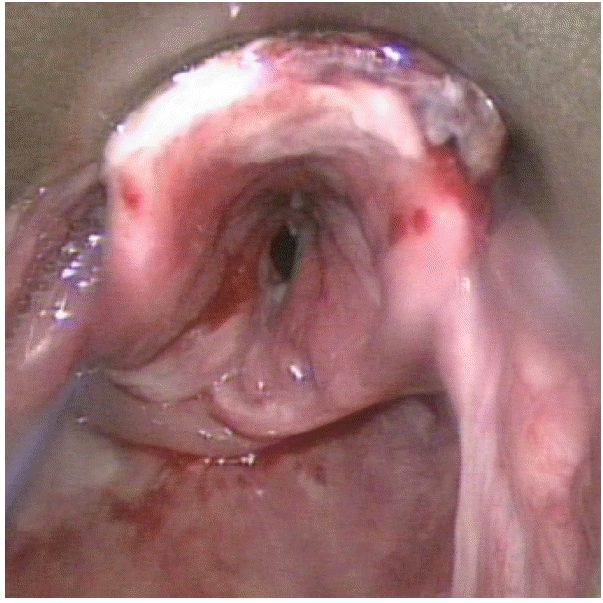
Upper airway obstruction resulting from acute mucosal injury induced by direct ingestion of sodium picosulfate/magnesium citrate powder
Article information
Abstract
A 59-year-old man presented to the emergency department with a chief complaint of sore throat after swallowing sodium picosulfate/magnesium citrate powder for bowel preparation, without first dissolving it in water. The initial evaluation showed significant mucosal injury involving the oral cavity, pharynx, and epiglottis. Endotracheal intubation was performed for airway protection in the emergency department, because the mucosal swelling resulted in upper airway compromise. After conservative treatment in the intensive care unit, he underwent tracheostomy because stenosis of the supraglottic and subglottic areas was not relieved. The tracheostomy tube was successfully removed after confirming recovery, and he was discharged 3 weeks after admission.
INTRODUCTION
Sodium picosulfate/magnesium citrate (SP/MC) powder has been widely used for colon cleansing because it has an acceptable taste and a small volume of liquid is required after dissolving it [1,2]. The effectiveness, safety, and ease of administration of this orange-flavored powder are well established [3,4]. Adverse effects are rare in clinical practice, although it may induce mucosal inflammation [5]. Here, we report the case of a patient who directly ingested SP/MC powder without first dissolving it in water, resulting in severe mucosal injury that extended from the oral cavity to the upper esophagus and caused airway compromise.
CASE REPORT
A 59-year-old man was brought to the emergency department (ED) in an ambulance with a chief complaint of sore throat. He had no significant medical history other than well-controlled hypertension. He was scheduled to undergo colonoscopy that day, and 2 h prior, he had unintentionally ingested a pack of undissolved SP/MC powder (Picolight powder; Pharmbio Korea Co., Seoul, Korea) that had been prescribed for bowel evacuation. Upon arrival in the ED, he was hemodynamically stable and did not have respiratory distress, but he complained of progressive hoarseness. The emergency physician noted gross mucosal injury of the oropharynx, and examination using a fiberoptic laryngoscope revealed diffuse mucosal swelling and erosion extending from the oral cavity to the epiglottis, which obstructed the view of the vocal cords (Fig. 1). There were no signs of injury at other sites. Laboratory test results and plain chest radiography findings were normal. Endotracheal intubation was performed because his respiratory status was predicted to deteriorate, although he was well oxygenated and ventilated at that time. He was admitted to the intensive care unit for further management. After admission to the intensive care unit, the upper gastrointestinal tract was examined using a flexible endoscope, and mucosal hyperemia, swelling, hemorrhage, and some necrotic changes extending from the oral cavity to the pharynx and epiglottis were observed. Mild hyperemia was noted in the esophagus (Fig. 2); the stomach was not affected.
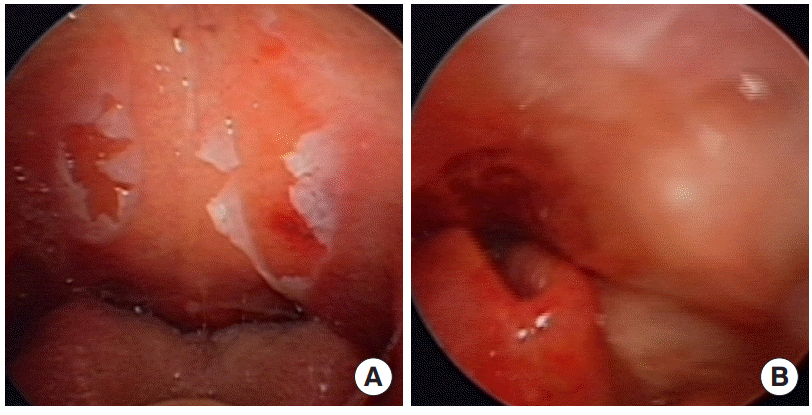
Fiberoptic laryngoscopic images showing mucosal injury of the oral cavity (A) and supraglottic area obstructing the view of the vocal cords (B).
Supportive therapy was maintained, including intravenous steroid administration to reduce airway swelling, and he was examined regularly to evaluate the airway status and assess the feasibility of extubation. Early tracheostomy was considered, but it was not performed in the first week after admission because he refused the procedure; therefore, we decided to wait for spontaneous recovery. However, on day 12 of hospitalization, fiberoptic examination and computed tomography of the neck revealed persistent stenosis of the supraglottic and subglottic areas (Fig. 3). Therefore, tracheostomy was performed on day 14 of admission, and remnant mucosal damage was still present (Fig. 4). The tracheostomy tube was successfully removed after confirming recovery, and the stoma was closed on day 21 of hospitalization. He was discharged the following day. He visited the outpatient clinic for follow-up 1 month after discharge, and no functional or structural sequelae were noted.
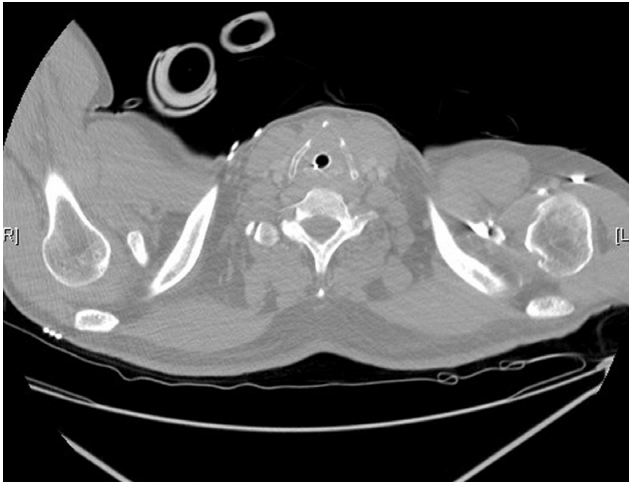
A computed tomography image showing swelling of the subglottic area surrounding the endotracheal tube.
DISCUSSION
SP/MC powder, composed of sodium picosulfate, magnesium oxide, and citric acid, is widely prescribed for bowel preparation. When the powder is dissolved in water, magnesium oxide and citric acid react to form magnesium citrate [6]. In solution, sodium picosulfate acts as a laxative resulting in augmented peristalsis, and magnesium citrate acts as an osmotic laxative and is not absorbed in the gastrointestinal tract [6,7]. Side effects of SP/MC are uncommon; however, it has been reported to induce nausea, vomiting, abdominal pain, dehydration, and electrolyte disturbances [5,8,9]. The most serious known adverse effect is severe electrolyte imbalance that includes hyponatremia, and rapid worsening of hyponatremia can lead to death [8,9].
To our knowledge, this is the first report of life-threatening upper airway mucosal damage induced by direct ingestion of SP/MC powder. Suh et al. [10] reported esophageal and gastric mucosal ulcers that resulted from the inadvertent ingestion of SP/MC powder. The mechanism of mucosal injury caused by SP/MC powder is uncertain. However, it may be related to direct thermal injury from heat generated when the powder mixes with a small volume of water or secretions. Citric acid may also cause caustic injury to the mucosa.
Considering that SP/MC powder is commonly used for bowel cleansing, ED physicians and those who prescribe SP/MC powder should be aware that serious mucosal injury can occur if the powder is directly ingested without dissolving it in water. To prevent this serious complication, thorough instructions and patient education are important. In particular, health care providers should emphasize that SP/MC powder should be dissolved in an adequate volume of water.
Notes
No potential conflict of interest relevant to this article was reported.
References
Article information Continued
Notes
Capsule Summary
What is already known
Sodium picosulfate/magnesium citrate is one of several colon cleansing agents and its serious side effects are uncommon.
What is new in the current study
Ingestion of undissolved powder of sodium picosulfate/magnesium citrate can cause life-threatening mucosal injury involving the upper airway.