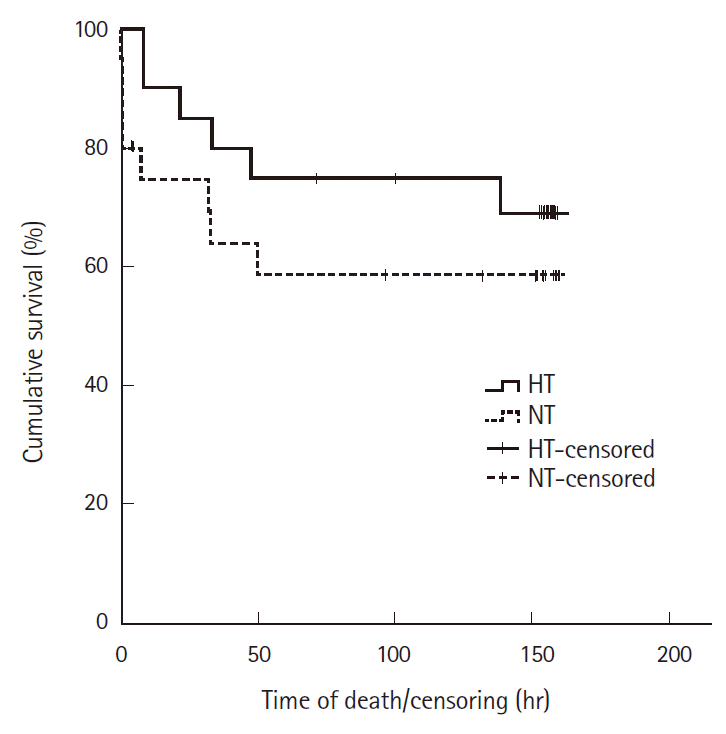INTRODUCTION
Mild therapeutic hypothermia has been evaluated for several clinical conditions, including cardiac arrest, myocardial infarction and stroke [1-5]. The Hypothermia Study Group demonstrated that mild hypothermia after cardiac arrest resuscitation increased favorable neurological outcome and reduced overall mortality following ventricular fibrillation [6]. More recently, Testori et al. [7] showed that mild hypothermia supported favorable neurological outcome and mortality following non-shockable rhythms. While the exact effects of hypothermia are unknown, a reduction in metabolic rate, inflammation and oxidative stress all may play a role. However, the role of cooling in burn treatment remains controversial. A large number of animal and clinical studies demonstrate improved outcomes with local surface cooling of burns at room temperature [8-41]. In contrast, Ofeigson [20,21] and Ofeigson et al.[22] showed that cooling of large scald burns in rats increased mortality. Subsequent studies in other animal populations also demonstrated similar outcomes. In addition data from trauma and surgical patients indicate that spontaneous hypothermia worsens outcomes [42-49]. As a result, burn specialists generally avoid excessive cooling in treatment protocols for fear of inducing hypothermia, skin necrosis, and worsening shock, among other negative outcomes.
A number of cytokines, such as interleukin (IL)-6, IL-1 beta, and tumor necrosis factor (TNF)-alpha, contribute to the pro-inflammatory state in thermal injury. TNF-alpha is thought to promote wound progression by delaying neutrophil apoptosis and inducing keratinocyte apoptosis [50]. Yeh et al. [51] found a significant difference in TNF-alpha serum levels between thermal injury survivors and non-survivors. In addition, burn injury is thought to induce the release of reactive oxygen and nitrogen species, further contributing to injury progression and the systemic inflammatory response syndrome [52-54]. These mechanisms also play a role in the pathogenesis of myocardial infarction, stroke and post cardiac arrest where hypothermia is thought to blunt these responses. As a result, we thought that mild controlled hypothermia might also have a beneficial role in burn induced injury.
Earlier animal models demonstrating worse outcome with hypothermia focused on superficial cooling and not core cooling. Additionally, hypothermia was neither controlled nor mild. We had previously shown that controlled mild hypothermia improved mortality in rats [55]. However, a major limitation to our earlier study was that the animals were not fluid resuscitated following burn injury. In a typical clinical scenario, aggressive early fluid resuscitation would be administered.
The current study was designed to assess the effects of mild therapeutic hypothermia in a large scald injury rat model in which the animals received fluid resuscitation. We hypothesized that controlled core cooling would improve overall mortality in a fluid resuscitated rat model of large scald burns.
METHODS
Study design
The study hypothesis was tested using a prospective randomized controlled experimental animal study. The study was conducted in an accredited laboratory animal research facility with approval by the Institutional Animal Care and Handling Committee. Housing and care for animals was managed under National Research Council guidelines [56].
Animals
Forty Sprague-Dawley rats weighing between 250-300 g were obtained from Charles River Laboratories in Wilmington, MA, USA. Animals were acclimated for 1-week prior to experimentation on standard rat chow while exposed to intermittent 12-hour cycles of light and dark.
Study protocol
Forty Sprague-Dawley rats were anesthetized with 40 mg/kg intramuscular ketamine and 5 mg/kg xylazine, with supplemental inhalational isoflurane 1%ŌĆō5% as needed. A single full-thickness scald burn covering 40% of the total body surface area (TBSA) was created on each rat using a Mason-Walker template placed in boiling water (100┬░C) for a period of 10 seconds [57].
Immediately after injury, the rats were fluid resuscitated. The fluid requirement was determined using the Parkland formula (4 mL/% TBSA burn/kg). Half of the fluid requirement was injected as normal saline into the peritoneal cavity following burn induction. The second half of the fluid requirement was injected into the peritoneal cavity eight hours later.
Induction of mild controlled hypothermia
The rats were randomized to a hypothermic (n=20) or normothermic (n=20) group. In the experimental group, mild hypothermia was induced by administering cooled (4┬░C) resuscitation fluids 30 minutes after burn injury. Following fluid resuscitation with cooled normal saline, the core temperature was maintained at 2┬░C below baseline by applying an ice or heat pack when necessary. Core temperature was monitored with a rectal thermometer. After two hours of hypothermia, the rats were re-warmed back to baseline temperature using a heating pad and received 0.5 mg/kg buprenorphine by intraperitoneal injection to control pain. We based the duration of hypothermia and rewarming on an earlier cooling study of acute lung injury on rats [58]. These durations of cooling and rewarming were also found to be effective in our previous animal study [55]. The rats were then monitored until recovery from anesthesia and returned to their cages. The wounds were left undressed, and additional buprenorphine was given intramuscularly as needed for pain.
Rats that appeared lethargic, lost>15% of pre-experiment weight, failed to eat, drink or ambulate were euthanized with CO2 inhalation. The rats were monitored until death or for a period of seven days, whichever was greater. The primary outcome was death.
Data analysis
Continuous data (e.g., temperature) are presented as a means with standard deviations. Categorical data (e.g., survival) are presented as counts and percentages. A Kaplan-Meier analysis and log-rank test was used to compare the difference in survival between the two groups. The primary outcome, survival, was compared between the two groups using the Žć2 or Fisher exact test. We tested assumptions of the temperature data for normality with the Shapiro-Wilk test, sphericity with the Mauchly test, and equality of variance with Levene test. The temperature profiles over time from baseline to 150 hours were then compared with repeated-measures analysis of variance, the between group factor being group membership. We included group/temperature interaction to the model to compare the shape of the temperature profile. A P-value of 0.05 was considered statistically significant. Data were analyzed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Based on our prior study, a sample of 20 burns in each group had 85% power to detect a difference of 20% in survival rates at seven days.
RESULTS
Full thickness burn injury was confirmed by hematoxylin and eosin stain (H&E) histology. The mean baseline temperature for the normothermic group was 35.3┬░C and for the hypothermic group was 35.2┬░C. The mean temperatures after the cooling period were 32.5┬░C for the hypothermic group and 35.6┬░C (P=0.03) for the normothermic group. The variation in core temperature during the two-hour recovery period is shown in Fig. 1.
Complete temperature data was available for 33 rats. Six normothermic and 1 hypothermic rats did not have complete temperature data and were excluded from the temperature analysis. Three normothermic rats were censored prior to the study endpoint. Two hypothermic rats were censored prior to the study conclusion. These rats were assumed to have survived to the endpoint in our analysis.
The number of rats that expired at each time interval were: at 1 hour, 4 normothermic and zero hypothermic animals; at 10 hours, 2 from each group; at 24 hours, zero normothermic and 1 hypothermic; at 48 hours, 2 normothermic and 2 hypothermic; 72 hours, 1 normothermic and 1 hypothermic animals; and at 120 hours, 1 normothermic and 1 hypothermic animals. There were no additional deaths after 120 hours. The mean survival times were 100 hours for the normothermic group (95% confidence interval [CI], 68 to 132) and 124 hours for the hypothermic group (95% CI, 98 to 150) as shown in Fig. 2. The mean time to survival of the hypothermic rats was not significantly greater than that of the normothermic rats (P=0.33).
DISCUSSION
Our results indicate that mild controlled hypothermia when induced with intraperitoneal fluid resuscitation did not significantly reduce mortality or prolong survival in our rat model of large scald burn injuries. These results were unexpected in view of a previous study from our laboratory, which demonstrated improved survival in non-fluid resuscitated rats with large scald burn injuries using similar hypothermic conditions [55].
There are several possible reasons why our study hypothesis was not confirmed in addition to the most obvious one, that systemic hypothermia is ineffective. In the current study, fluid resuscitation was given as two large intraperitoneal boluses separated by eight hours. In contrast, continuous intravenous fluid resuscitation is used in the clinical scenario. Overzealous and rapid fluid administration may have resulted in the abdominal compartment syndrome or volume overload that may have eliminated any possible beneficial effects of mild therapeutic hypothermia. This may be similar to the phenomenon of ŌĆ£fluid creepŌĆØ described in humans when excessive intravenous fluids are administered [59].
It is also possible that the duration of hypothermia may have been too short to result in any benefit. Indeed, after cardiac arrest in humans much longer periods of hypothermia are maintained. We chose a two-hour period based on the success in prior rat studies [55,58]. It is also possible that the method and speed of rewarming after hypothermia were inappropriate despite success with similar methods in the prior un-resuscitated animal model.
A recent large study of survivors of cardiac arrest demonstrated that maintaining a temperature of 36┬░C was as effective as inducing a mild hypothermia of 32┬░CŌĆō34┬░C [60]. Thus it is also possible that a different target temperature may have been more effective than the one studied by our group. It is also possible that our study was underpowered to detect small differences in survival that still might be clinically significant.
In addition to the limitations noted above with regards to the methods of fluid resuscitation, cooling and rewarming, several other limitations should be noted. Our study may have been underpowered to detect any difference in the primary outcome, mortality. Most importantly, while the rat scald burn model has been commonly used, it is unclear how representative it is of human burns. Thus our results may not generalize to the clinical scenario.
In conclusion, induction of brief mild hypothermia by administration of intraperitoneal fluid boluses does not significantly prolong survival in a resuscitated rat model of large scald burns. Further studies should consider the use of continuous intravenous fluid administration as well as extension of the cooling and warming periods before abandoning mild therapeutic hypothermia for burns.