AbstractObjectiveMigraine headache is a chronic and disabling condition in adults. Some studies have investigated the efficacy of sodium valproate in the treatment of acute migraine, but the effectiveness and tolerability of intravenous valproate as abortive therapy remains unclear. This study aimed to evaluate the effects of sodium valproate and dexamethasone in the treatment of acute migraine.
MethodsWe conducted a double-blind randomized clinical trial including 90 patients aged 18 to 65 years with acute migraine headache but no aura. Patients were randomized to receive intravenous dexamethasone (8 mg) or sodium valproate (400 mg) diluted into 4 mL of normal saline. The primary outcome measure was pain relief after 0.5, 1, 3, or 6 hours after administration. The secondary outcome criteria were the associated symptom recovery, rate of headache recurrence after 24 hours, and medication side effects. Pearson’s chi square and the t-test were employed in the data analysis.
ResultsOf the 90 patients, 80 were investigated. The percentage of headache improvement at 0.5 hours after treatment was 55% and 67.5% in the sodium valproate and dexamethasone groups, respectively. Before-treatment and 0.5 hour after treatment pain severity visual analog scale scores were 9.05±0.90 and 3.8±3.09 in the sodium valproate group and 8.92±0.79 and 3.10±2.73 in the dexamethasone group, respectively. There were no significant intergroup differences.
INTRODUCTIONMigraine headache is a chronic and disabling primary headache in adults that constitutes 1% to 2% of all emergency department admissions [1]. The exact prevalence of migraine is unknown and varies throughout the world. In Iran, the prevalence of migraine is reportedly 18.11% in adults in Tehran and 6% in Yazad city, while the lowest prevalence (1.7%) was reported in primary-school children in Shiraz [2-4]. This disorder presents with attacks of moderate to severe throbbing headaches unilaterally associated with nausea and/or vomiting, photophobia, and phonophobia [5].
Medications used to treat acute attacks include triptans, ergotamine, nonsteroidal anti-inflammatory drugs, intravenous fluids, antiemetic, opioids, neuroleptics, and dexamethasone [6-10]. Studies have shown different effects of dexamethasone in the treatment of migraine headaches [11,12]. Some studies reported that dexamethasone is significantly effective for treating acute migraine and reducing the risk of early headache recurrence due to its anti-inflammatory effects without dangerous side effects, even if administrated as a high single dose [13-21]. Some studies have investigated the efficacy of sodium valproate with different doses in the treatment of acute migraine, and its efficacy has been compared with other drugs such as sumatriptan, ketorolac, metoclopramide, or dexamethasone [22-26]. These studies and other surveys have reported different results [27-30]. In different regions of Iran, two studies had compared intravenous sodium valproate and dexamethasone for the treatment of acute migraine with different samples and dosages [23,24].
Sodium valproate is an antiepileptic agent that is used prophylactically and more recently for the treatment of acute migraine. Sodium valproate increases γ-amino butyric acid levels in the central nervous system and diminishes firing rates of serotonergic cells. This drug directly affects the neuronal cell membrane and limits the repetitive firing rate of the sodium and calcium current. This effect occurs rapidly and can explain the influence of sodium valproate in abortive therapy [31-34]. The influence of sodium valproate as abortive therapy remains unclear, and US Food and Drug Administration only recently approved its use for abortive therapy; additionally, studies have reported the different effects of intravenous sodium valproate for the treatment of acute migraine [29,34,35]. This study aimed to compare the effects of intravenous sodium valproate and other common drugs (dexamethasone) used in the treatment of acute migraine.
METHODSTrial designThis double-blinded randomized clinical trial (registration no. IRCT2014022116666N1) included adult patients (18 to 65 years old) with migraine headache without aura who fulfilled the international classification of headache disorders, 3rd edition (beta version), diagnostic criteria for migraine [5].
Settings and participantsThis study was performed in the clinical research development unit of a university referral emergency department in Sari city, Mazandaran province, Iran, between October 2014 and June 2016. The inclusion criteria included age 18 to 65 years, history of migraine type headache without aura for at least 1 year prior to the study, moderate to severe headache persisting for less than 6 days with a visual analog scale (VAS) score >7, normal vital signs, and normal neurological exam findings. Patients with abnormal vital signs, renal failure, hepatic disease, heart disease, active peptic ulcer disease, or diabetes mellitus were excluded from the study. Participants with allergies to dexamethasone and sodium valproate or who were pregnant or breastfeeding were excluded from the study. Patients who had taken nonsteroidal anti-inflammatory drugs during the previous 24 hours or valproate during the previous 2 weeks were not included in the study.
Sample size, randomization, and blindingAccording to the results of previous clinical trials [36,37] with 80% power and a confidence interval of 95%, a total sample size of 38 was calculated in each group. Participation in the study was discontinued when a total of 80 patients were enrolled. A CONSORT (Consolidated Standards of Reporting Trials) flow diagram illustrates the flow of participants enrolling into the study (Fig. 1). Block randomization was used for recruiting participants. Randomization was accomplished using a computerized random number table. The drugs were placed in envelopes randomly labeled with a serial number from 1,000 to 2,000 using the RANDBETWEEN function in Excel (Microsoft, Redmond, WA, USA). Drugs were prepared in the same syringe and injected by a nurse who was not part of the study. Both the investigator (emergency physician and neurologist) and patient remained blinded to the medication delivered until the code was determined at the end of the study. The patients were selected by a general physician after physical examination by a neurologist and meeting the eligibility criteria and recruited for the study. Written informed consent was obtained from all patients and the study proposal was approved by the Regional Ethics Committee of Mazandaran University of Medical Sciences (code no. 92.12.14). Prior to the drug administration, all participants filled out a questionnaire that included demographic information, headache duration and severity, and associated symptoms (photophobia, phonophobia, nausea, and vomiting).
Intervention and outcomesAfter patient randomization into two groups, dexamethasone 8 mg was intravenously administered to the control group and sodium valproate 400 mg (Depakine; Sanofi Aventis, Paris, France) diluted into 4 mL of normal saline was intravenously administered to the intervention group. Pain severity and other associated symptoms such as nausea or vomiting, photophobia, and phonophobia, and the presence of adverse effects were assessed at baseline and at 0.5, 1, 3, and 6 hours and as well the rate of recurrent headache at 24 hours after receiving the drugs. The primary outcome criteria were pain relief (no or mild pain and a VAS score ≤3) after 0.5, 1, 3, and 6 hours after receiving the drug. Secondary outcome criteria were associated symptom recovery over 24 hours, headache recurrence rate after 24 hours, and medication side effects.
Measuring headache severityHeadache severity was assessed on the basis of VAS score and pain intensity. The VAS scores range from 0 (no pain) to 10 (worst pain). The patients also recorded pain intensity on a four-point categorical scale (0=no pain, 1=mild pain, 2=moderate pain, and 3=severe pain) [38]. Pain monitoring was continued using the VAS score and pain intensity by the neurologist throughout the management. The goal of treatment was no or mild pain.
Statistical analysisPatients’ demographic data and the variables mentioned earlier were entered into the IBM SPSS Statistics ver. 20 (IBM Corp., Armonk, NY, USA). Pearson’s chi-square test and the t-test were used to determine the relationship between demographic information and drugs, while the independent t-test and chi-square test were used to evaluate post-treatment pain severity in the two groups. Kaplan-Meier survival analysis and log-rank test were utilized to estimate time to headache relief and treatment success between the two groups within the first 6 hours. Treatment success was defined as decreased pain severity with a VAS score ≤3 as well as no or mild pain. The statistical significance level was set at P<0.05.
RESULTSNinety patients with acute migraine headache were screened for inclusion in this study. Seven patients did not meet the inclusion criteria and were excluded. Three participants did not fill out the consent form. A total of 80 patients were enrolled and analyzed. All of these patients continued follow up. There were 34 women and 6 men (mean age, 33.9±9.5 years) in the control group (dexamethasone) and 31 women and 9 men (mean age, 33.38± 9.15 years) in the intervention group (sodium valproate). The patients’ demographic information is shown in Table 1. There were no significant intergroup differences in demographic features and the two groups were similar. All participants mentioned photophobia and phonophobia but only one patient did not mention phonohobia in the valproate group. Seven patients (17.5%) in the dexamethasone group and five patients (12.5%) in the sodium valproate group did not report vomiting. There was no intergroup difference in associated symptoms.
The mean VAS pain severity scores prior to drug administration in the dexamethasone and valproate groups were 8.92±0.79 (95% confidence interval, 8.68 to 9.18) and 9.05±0.9 (95% confidence interval, 8.76 to 9.34), respectively. Thirty minutes after the therapeutic intervention, the pain severity was significantly reduced in the dexamethasone (3.10±2.73) and sodium valproate (3.8±3.09) groups, but there was no significant intergroup difference (Table 2).
In terms of headache relief efficacy, the differences between values 0.5, 1, 3, and 6 hours after treatment and those before treatment were statistically significant (P<0.001) (Table 3). Table 4 shows the reduction in pain intensity after versus before treatment according to the 4-point scale. At 0.5 hours after administration, the dexamethasone and valproate groups showed a successful reduction in pain severity in 22 (67.5%) and 27 (55%) of the patients, respectively, but there were no significant intergroup differences (P=0.17). There were also no statistically significant intergroup differences in age, sex, disease duration or improvement of headache. The Kaplan-Meier curve demonstrated the time to pain relief onset and success rates in both groups (Fig. 2). Any patient with a VAS score ≤3 after treatment was considered a treatment success. The log-rank test demonstrated no inter-group difference in therapeutic effect (χ2 value [1, 80]=2.018; P=0.155). Overall, 36 patients (90%) in the valproate group and 39 patients (97.5%) in the dexamethasone group showed pain improvement up to 6 hours after drug administration. The patients remained under observation in the emergency department for 24 hours. Six patients (15%) from the sodium valproate group and two patients (5%) from the dexamethasone group required rescue therapy 6 hours after treatment. Four patients (10%) in the valproate group and one patient (2.5%) in the dexamethasone group showed no improvement 6 hours after administration. The frequency of recurrent headache at 24 hours after treatment was the same in both groups (7.5%). Migraine associated symptoms including photophobia, phonophobia, and nausea or vomiting were significantly improved in the two groups. No adverse effects were noted in the dexamethasone group, while only one patient in the valproate group had an adverse effect consisting of anxiety, unrest, and shortness of breath that improved 2 hours after treatment.
DISCUSSIONThis randomized clinical trial demonstrated that dexamethasone and sodium valproate effectively treated acute migraine headache. More patients in the dexamethasone than sodium valproate group recovered, but the difference was not statistically significant. In other words, the drugs demonstrated the same efficacy for treating acute migraine headache. Similar studies performed in Iran during 2013 and 2015 showed that the two drugs had the same effects on headache relief [23,24]. In the present study, the headache relief rates in the sodium valproate and dexamethasone groups were 55% and 67.5% in the first 0.5 hour, respectively. In other studies, the percentage of pain relief after 1 hour in patients receiving sodium valproate was reported as 25% [39] and 53.3% [28]. There was no significant association between the two groups in terms of demographic factors and recovery rate in the first 0.5 hour.
Foroughipour et al. [24] reported the duration of headache relief in the sodium valproate and dexamethasone groups as 292 and 270 minutes in 26% and 33% of patients, respectively. In that study, the sample size was small and the patients received therapeutic dosages higher than those used in our study. Unfortunately, the therapeutic dosage of sodium valproate for acute migraine headache has yet to be determined. In several studies, the therapeutic dosage of valproate was variable (400 to 1,200 mg) and the drug was diluted in normal saline (50 to 200 mL) [23-26]. In this study, both drugs were administered in a single dose diluted in a small amount of normal saline (4 mL) to avoid a confounding effect of normal saline, which is able to reduce headache via hydration [10,40]. Limdi et al. [41] reported the safety of a rapid infusion of undiluted sodium valproate in the treatment of epilepsy. The results of the present study demonstrated that the administration of a low dose of dexamethasone (8 mg) and sodium valproate (400 mg) diluted in 4 mL of normal saline can significantly decrease headache pain.
Bakhshayesh et al. [28] reported that sodium valproate was more effective than metoclopramide and sumatriptan at providing headache relief during the 2 hours post-treatment, whereas Rahimdel et al. [25] reported that intravenous sodium valproate and subcutaneous sumatriptan have similar abilities to control acute migraine attacks. Edwards et al. [33] reported equal effectiveness in headache improvement after treatment with sodium valproate compared with dihydroergotamine and metoclopramide. Tanen et al. [34] compared the efficacy of intravenous sodium valproate versus prochlorperazine and demonstrated that the former was less effective at decreasing pain or nausea; however, patients were followed for the first hour and also received rescue therapy, which is a confounding variable.
In this study, the rates of headache recurrence after 24 hours were very low in both groups. Foroughipour et al. [24] demonstrated that relapse of headache occurred in 68.4% of the sodium valproate group and 66.7% of the dexamethasone group within 72 hours after injection. In addition, Ghaderibarmi et al. [26] demonstrated that sodium valproate is more effective than sumatriptan at reducing pain and preventing headache recurrence. One study reported that intravenous dexamethasone was less effective at preventing acute migraine headache relapse, while another study revealed a reduced incidence of severe recurrent headache after dexamethasone infusion [30,42]. The number of cases in both studies was lower than that in ours, but both followed up their patients for 72 hours. In this study, the patients were assessed for 24 hours. It seems that a longer follow-up is required to evaluate recurrence.
In our research, sodium valproate and dexamethasone were equally effective at improving migraine-associated symptoms. All of our patients reported improved pain at the same post-infusion duration. This finding is consistent with those of other studies [25,26], but Foroughipour et al. [24] reported that dexamethasone was more effective than sodium valproate at treating the associated symptoms. The improvement of associated symptoms appears to be dependent on their severity and duration prior to treatment. Further investigations of this issue are required. In the current study, only one patient in the sodium valproate group reported a mild side effect. Moreover, other studies reported no serious adverse effect after sodium valproate or dexamethasone treatment [23-26]. The safety and tolerability of intravenous sodium valproate and dexamethasone for treating acute migraine attacks have been demonstrated in several investigations [16,27,35,39,42]. To the best of our knowledge, the study by Shahien et al. [43] is the only one to report serious side effects of intravenous sodium valproate, which can be attributed to the high dose used (900 to 1,200 mg).
In conclusion, the rapid intravenous injection of a single dose of sodium valproate 400 mg had similar efficacy to that of dexamethasone at improving acute migraine headache without causing serious side effects. Moreover, these medications significantly improved the patients’ migraine-associated symptoms.
ACKNOWLEDGMENTSWe would like to appreciate the Vice Chancellor of Research and Technology of Mazandaran University of Medical Sciences for approval and financial support of this trial. The authors would like to thank the emergency physicians at Boo Ali Sina Hospital and also all of the patients that participated in this study. This study was Mahdiye Tavakoli’s doctoral dissertation towards graduation in general medicine.
REFERENCES1. Talabi S, Masoumi B, Azizkhani R, Esmailian M. Metoclopramide versus sumatriptan for treatment of migraine headache: a randomized clinical trial. J Res Med Sci 2013; 18:695-8.
2. Shahbeigi S, Fereshtehnejad SM, Mohammadi N, et al. Epidemiology of headaches in Tehran urban area: a population-based cross-sectional study in district 8, year 2010. Neurol Sci 2013; 34:1157-66.
3. Momayyezi M, Fallahzadeh H, Momayyezi M. Prevalence of migraine and tension-type headache in Yazd, Iran. Zahedan J Res Med Sci 2015; 17:e966.
4. Ayatollahi SM, Khosravi A. Prevalence of migraine and tension-type headache in primary-school children in Shiraz. East Mediterr Health J 2006; 12:809-17.
5. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders 3rd edition (beta version). Cephalalgia 2013; 33:629-808.
6. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults. Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78:1346-53.
7. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754-62.
8. Tfelt-Hansen P, Block G, Dahlof C, et al. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000; 20:765-86.
9. Rizzoli PB. Acute and preventive treatment of migraine. Continuum (Minneap Minn) 2012; 18:764-82.
10. Gupta S, Oosthuizen R, Pulfrey S. Treatment of acute migraine in the emergency department. Can Fam Physician 2014; 60:47-9.
11. Rowe BH, Colman I, Edmonds ML, Blitz S, Walker A, Wiens S. Randomized controlled trial of intravenous dexamethasone to prevent relapse in acute migraine headache. Headache 2008; 48:333-40.
12. Fiesseler FW, Shih R, Szucs P, et al. Steroids for migraine headaches: a randomized double-blind, two-armed, placebo-controlled trial. J Emerg Med 2011; 40:463-8.
13. Soleimanpour H, Ghafouri RR, Taheraghdam A, et al. Effectiveness of intravenous dexamethasone versus propofol for pain relief in the migraine headache: a prospective double blind randomized clinical trial. BMC Neurol 2012; 12:114.
15. Singh A, Alter HJ, Zaia B. Does the addition of dexamethasone to standard therapy for acute migraine headache decrease the incidence of recurrent headache for patients treated in the emergency department? A meta-analysis and systematic review of the literature. Acad Emerg Med 2008; 15:1223-33.
16. Bigal M, Sheftell F, Tepper S, Tepper D, Ho TW, Rapoport A. A randomized double-blind study comparing rizatriptan, dexamethasone, and the combination of both in the acute treatment of menstrually related migraine. Headache 2008; 48:1286-93.
17. Ropper AH, Samuels MA, Klein JP. Adams and Victor’s principles of neurology. 10th ed. New York, NY: MC Graw Hill; 2014.
18. Daroff RB, Fenichel GM, Jankovic J, Mazziotta JC. Neurology in clinical practice. 6th ed. New York, NY: Elsevier; 2012.
19. Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336:1359-61.
20. Baden EY, Hunter CJ. Intravenous dexamethasone to prevent the recurrence of benign headache after discharge from the emergency department: a randomized, double-blind, placebo-controlled clinical trial. CJEM 2006; 8:393-400.
21. Hardman JG, Limbird LE. Goodman and Gilman’s the pharmacological basis of therapeutics. New York, NY: MC Graw Hill; 2001.
22. Friedman BW, Garber L, Yoon A, et al. Randomized trial of IV valproate vs metoclopramide vs ketorolac for acute migraine. Neurology 2014; 82:976-83.
23. Mazaheri S, Poorolajal J, Hosseinzadeh A, Fazlian MM. Effect of intravenous sodium valproate vs dexamethasone on acute migraine headache: a double blind randomized clinical trial. PLoS One 2015; 10:e0120229.
24. Foroughipour M, Ghandehari K, Khazaei M, Ahmadi F, Shariatinezhad K, Ghandehari K. Randomized clinical trial of intravenous valproate (orifil) and dexamethasone in patients with migraine disorder. Iran J Med Sci 2013; 38:150-5.
25. Rahimdel A, Mellat A, Zeinali A, Jafari E, Ayatollahi P. Comparison between intravenous sodium valproate and subcutaneous sumatriptan for treatment of acute migraine attacks: double-blind randomized clinical trial. Iran J Med Sci 2014; 39:171-7.
26. Ghaderibarmi F, Tavakkoli N, Togha M. Intravenous valproate versus subcutaneous sumatriptan in acute migraine attack. Acta Med Iran 2015; 53:633-6.
27. Friedman BW, Greenwald P, Bania TC, et al. Randomized trial of IV dexamethasone for acute migraine in the emergency department. Neurology 2007; 69:2038-44.
28. Bakhshayesh B, Seyed Saadat SM, Rezania K, Hatamian H, Hossieninezhad M. A randomized open-label study of sodium valproate vs sumatriptan and metoclopramide for prolonged migraine headache. Am J Emerg Med 2013; 31:540-4.
29. Frazee LA, Foraker KC. Use of intravenous valproic acid for acute migraine. Ann Pharmacother 2008; 42:403-7.
30. Donaldson D, Sundermann R, Jackson R, Bastani A. Intravenous dexamethasone vs placebo as adjunctive therapy to reduce the recurrence rate of acute migraine headaches: a multicenter, double-blinded, placebo-controlled randomized clinical trial. Am J Emerg Med 2008; 26:124-30.
32. Stillman MJ, Zajac D, Rybicki LA. Treatment of primary headache disorders with intravenous valproate: initial outpatient experience. Headache 2004; 44:65-9.
33. Edwards KR, Norton J, Behnke M. Comparison of intravenous valproate versus intramuscular dihydroergotamine and metoclopramide for acute treatment of migraine headache. Headache 2001; 41:976-80.
34. Tanen DA, Miller S, French T, Riffenburgh RH. Intravenous sodium valproate versus prochlorperazine for the emergency department treatment of acute migraine headaches: a prospective, randomized, double-blind trial. Ann Emerg Med 2003; 41:847-53.
35. Waberzinek G, Markova J, Mastik J. Safety and efficacy of intravenous sodium valproate in the treatment of acute migraine. Neuro Endocrinol Lett 2007; 28:59-64.
36. Foroughipour M, Petramfar P, Saeidi M, Akbarnezhad MA, Ebrahimzadeh S. Efficacy of intravenous Dexamethasone in treatment of acute migraine headache: a randomized clinical trial running title. Iran J Otorhinolaryngol 2008; 19:27-31.
37. Mathew NT, Kailasam J, Meadors L, Chernyschev O, Gentry P. Intravenous valproate sodium (depacon) aborts migraine rapidly: a preliminary report. Headache 2000; 40:720-3.
38. Sjaastad O, Fredriksen TA, Petersen HC, Bakketeig LS. Grading of headache intensity: a proposal. J Headache Pain 2002; 3:117-27.
39. Leniger T, Pageler L, Stude P, Diener HC, Limmroth V. Comparison of intravenous valproate with intravenous lysine-acetyl-salicylic acid in acute migraine attacks. Headache 2005; 45:42-6.
41. Limdi NA, Knowlton RK, Cofield SS, et al. Safety of rapid intravenous loading of valproate. Epilepsia 2007; 48:478-83.
Fig. 1.CONSORT (Consolidated Standards of Reporting Trials) flow diagram.
Ten patients were excluded because they did not meet inclusion or exclusion criteria. In general, 80 participants were analyzed.
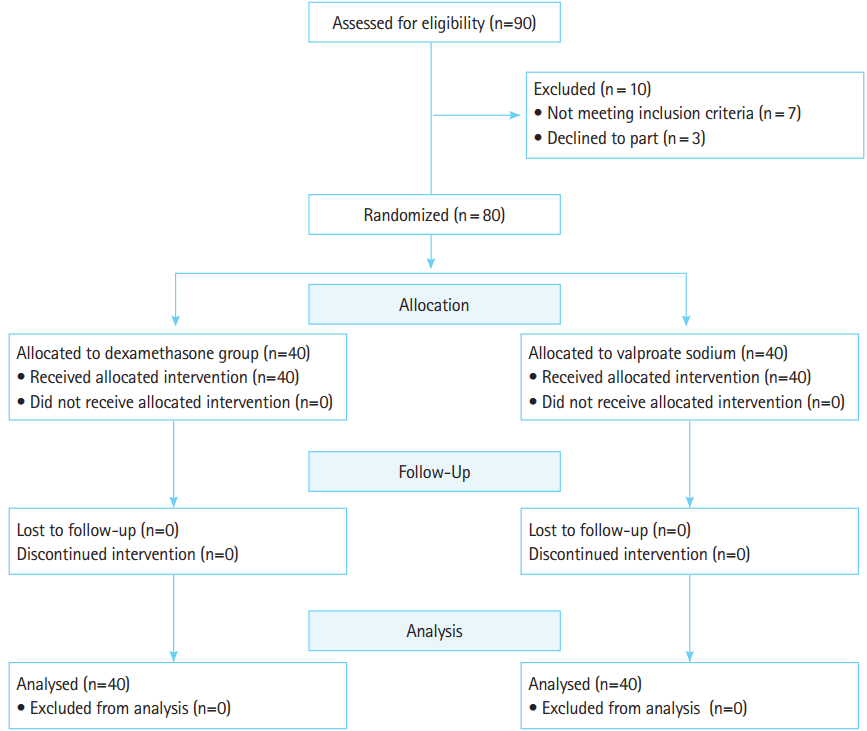
Table 1.Patient demographics Table 2.Visual analog scale pain scores before versus after treatment by group Table 3.Effect of each drug on headache relief by visual analog scale score
Table 4.Significant improvement of pain after treatment based on four-point scalea)
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||