AbstractObjectiveTherapeutic hypothermia (TH) has become the standard strategy for reducing brain damage in the postresuscitation period. The aim of this study was to investigate current TH performance and outcomes in out-of-hospital cardiac arrest (OHCA) survivors using data from the Korean Hypothermia Network (KORHN) registry.
MethodsWe used the KORHN registry, a web-based multicenter registry that includes 24 participating hospitals throughout the Republic of Korea. Adult comatose OHCA survivors treated with TH between 2007 and 2012 were included. The primary outcomes were neurological outcome at hospital discharge and in-hospital mortality. The secondary outcomes were TH performance and adverse events during TH.
ResultsA total of 930 patients were included, of whom 556 (59.8%) survived to discharge and 249 (26.8%) were discharged with good neurologic outcomes. The median time from return of spontaneous circulation (ROSC) to the start of TH was 101 minutes (interquartile range [IQR], 46 to 200 minutes). The induction, maintenance, and rewarming durations were 150 minutes (IQR, 80 to 267 minutes), 1,440 minutes (IQR, 1,290 to 1,440 minutes), and 708 minutes (IQR, 420 to 900 minutes), respectively. The time from the ROSC to coronary angiography was 1,045 hours (IQR, 121 to 12,051 hours). Hyperglycemia (46.3%) was the most frequent adverse event.
INTRODUCTIONMany cardiac arrest survivors remain comatose or die even after achieving return of spontaneous circulation (ROSC) [1]. Therapeutic hypothermia (TH) prevents neurologic injury from mitochondrial damage, cellular membrane damage, intracellular acidosis, the formation of oxygen free radicals, and increased excitotoxicity after ischemia–reperfusion injury related to cardiac arrest [2-5]. The American Heart Association (AHA) recommends TH (target temperature, 32°C to 34°C; duration, 12 to 24 hours) as class I for out-of-hospital cardiac arrest (OHCA) survivors with ventricular fibrillation or pulseless ventricular tachycardia and class IIb for OHCA survivors with non-shockable rhythm or in-hospital cardiac arrest [6]. However, many TH-related issues have yet to be sufficiently resolved. The effectiveness of TH for in-hospital cardiac arrest survivors or cardiac arrest survivors with non-shockable rhythm as well as the optimal TH target temperature and duration have not yet been proven [7-12].
The etiology of cardiac arrest, incidence of shockable rhythm, emergency medical system, policy of post-cardiac arrest care, and cardiac arrest outcomes differ among countries [13-17]. Therefore, we should identify the current TH status of OHCA survivors in Korea to suggest recommendations for TH suitable to our circumstances. The purpose of the present study was to investigate the current status of post-cardiac arrest care, including TH, and patients outcome using data from a multicenter registry in Korea.
METHODSPatients and registryThe Korea Hypothermia Network (KORHN) managed a web-based retrospective registry of cases of OHCA treated with TH that aimed to improve post-cardiac arrest care quality and outcomes. Adult (≥18 years) comatose patients treated with TH between January 2007 and December 2012 were included. Cases of cardiac arrest from trauma or stroke or that occurred in the hospital were excluded. Each principal investigator of 24 participating hospitals reviewed the hospital records of OHCA survivors treated with TH and entered their baseline characteristics, comorbidities, prehospital cardiac arrest characteristics, respiratory and circulatory status after ROSC, circulatory support, neurological status and exam results, TH practice, incidence of complications, cerebral performance category scale at discharge, and mortality rates. Three clinical research associates monitored the data and helped qualify it by sending queries to the investigators. Finally, a data manager checked the data and decided whether to accept or revise it.
Data collectionData of cardiac arrest, age, gender, comorbidities (coronary heart disease, congestive heart failure, stroke, hypertension, diabetes mellitus, lung disease, renal impairment, liver cirrhosis, and malignancy), witness of collapse, bystander cardiopulmonary resuscitation (CPR), first monitored rhythm (ventricular fibrillation, pulseless ventricular tachycardia, asystole, pulseless electrical activity, and unknown), etiology of cardiac arrest (cardiac, submersion, drug, asphyxia, exsanguination, and other non-cardiac diseases), coronary reperfusion and circulatory support (coronary angiography, percutaneous coronary intervention, coronary artery bypass graft, extracorporeal bypass, intra-aortic balloon pump, and continuous renal replacement therapy), time from ROSC to coronary angiography, time from collapse to ROSC, serum glucose after ROSC, Glasgow Coma Scale score after ROSC, time from ROSC to start of TH, time from start of TH to achieving the target temperature, maintenance duration, rewarming duration, target temperature, shock, TH method (blanket, ice bag, adhesive pad, garment, fan, linen, cold saline, intravascular catheter, lavage, and extracorporeal membrane oxygenation), core temperature monitoring site, complications (overcooling, bradycardia, hypokalemia, hyperglycemia, bleeding, hypotension, hyperthermia, hyperkalemia, hypoglycemia, seizure, pneumonia, and sepsis), and Cerebral Performance Category (CPC) score at discharge were collected.
The first monitored rhythm (collected by the emergency medical service or in the emergency department) was recorded. The time from collapse to ROSC was defined as the time from witness or detection of collapse to ROSC. Complications were defined as follows: overcooling (<32°C), hyperthermia (≥38°C), bradycardia (<40 beats/min), hypokalemia (≤3.0 mEq/L), hyperkalemia (≥5.0 mEq/L), hypoglycemia (<80 mg/dL), hyperglycemia (≥180 mg/dL), hypotension (systolic blood pressure [SBP]<90 mmHg, mean arterial pressure [MAP]<60 mmHg for at least 30 minutes or the need for supportive measures to maintain a SBP>90 mmHg or MAP>60 mmHg), seizure (either clinically involuntary movement or epileptiform discharge on an electroencephalogram), or pneumonia (new or progressive consolidation on the chest radiograph, fever, leukocytosis, and the presence of purulent tracheobronchial secretions).
Primary and secondary outcomesThe primary outcomes were neurologic outcome and survival assessed using the CPC score at hospital discharge according to the recommendations for outcome assessment in comatose cardiac arrest survivors and recorded as CPC 1 (good performance), CPC 2 (moderate disability), CPC 3 (severe disability), CPC 4 (vegetative state), and CPC 5 (brain death or death) [18]. Neurological outcome was dichotomized as either good (CPC 1 or 2) or poor (CPC 3–5). The secondary outcomes were practical TH status and its related complications.
Statistical analysisCategorical variables are given as frequencies and percentages. Comparisons of categorical variables were performed using the χ2 test or Fisher exact test as appropriate. Continuous variables are given as median values with interquartile ranges. The Mann-Whitney U-test was conducted to compare continuous variables. The Jonckheere-Terpstra test was used to analyze trends of non-normally distributed variables. Data were analyzed using PASW/SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). Significance was set at values of P<0.05.
RESULTSBaseline characteristicsA total of 930 OHCA survivors were treated with TH, and the fill-up rate of data record entry of each case was 99.1% (IQR, 98.1% to 100%). The median number of registered case at each hospital was 26 (IQR, 11 to 51). The maximal and minimal registered numbers of case were 171 and 2, respectively. Six hospitals started offering TH prior to 2007, two since 2009, six since 2010, nine since 2011, and one since 2012. Fig. 1 shows the geographical distribution of the 24 participating hospitals. A total of 39, 49, 75, 117, 274, and 375 OHCA survivors were treated with TH yearly between 2007 and 2012.
The patients’ clinical characteristics are shown in Table 1. The median age was 58 years (IQR, 46 to 70 years) and 650 patients (69.9%) were men. Patients with a good neurological outcome were significantly younger (P<0.001) and more likely to be male (P=0.001). Hypertension and diabetes mellitus were the most frequent comorbidities. Patients with stroke, hypertension, diabetes mellitus, lung disease, and renal impairment were more likely to have a poor neurological outcome. A total of 622 collapses (66.9%) were witnessed, while 281 OHCA survivors (30.2%) received bystander CPR. Shockable rhythms were delivered to 243 patients (26.1%), and non-shockable rhythms were delivered to 653 patients (70.2%). There were 564 cases (60.6%) of presumed cardiac etiology. A good neurological outcome was associated with witness of collapse, bystander CPR, shockable rhythm, and cardiac etiology (Table 1). Shorter time from collapse to ROSC and lower glucose after ROSC were associated with good neurological outcome (P<0.001).
Neurological outcome and survival according to first monitored rhythmOf the total 930 patients, 556 (59.8%) survived to discharge and 249 (26.8%) were discharged with good neurological outcomes. Of the 243 patients who had experienced OHCA with shockable rhythm, 202 (83.1%) survived to discharge and 147 (60.5%) were discharged with good neurological outcomes. Of the 653 patients with OHCA and non-shockable rhythm, 327 (50.1%) survived to discharge and 87 (13.3%) were discharged with good neurological outcomes. Fig. 2 shows the frequencies of neurological outcome and survival according to first monitored rhythm.
TH practice and complications
Table 2 shows the TH practice following ROSC. The median time from collapse to start of TH was 101 minutes (IQR, 46 to 200 minutes), and that of patients with a poor neurological outcome was significantly shorter (P=0.036). The target temperature of 33°C was most frequent. The median time from TH start to achieving the target temperature was 150 minutes (IQR, 80 to 267 minutes) and was significantly shorter in patients with a poor neurological outcome (P<0.001). The median rewarming duration was 708 minutes (IQR, 420 to 900 minutes). The time from ROSC to TH start tended to decline (P=0.002) and the rewarming duration tended to increase (P<0.001) over time (Table 3). The rectum was the most frequently (61.4%) monitored for core temperature, and dual sites were monitored in 84 patients (9.0%).
Table 4 shows the incidence of complications during TH. Hyperglycemia, hypotension, seizure, overcooling, sepsis, and hyperkalemia were significantly more frequent in patients with poor neurological outcomes.
Cardiac care, circulatory supportive therapy, and TH methods
Table 5 shows the incidence of coronary reperfusion therapy and circulatory supportive therapies. Cardiogenic shock developed in 293 patients (31.5%). Coronary angiography was performed in 236 patients (25.4%), while percutaneous coronary intervention was performed in 86 patients (9.2%). The median time from ROSC to angiography was 1,045 hours (IQR, 121 to 12,051 hours) and tended to decline (P<0.001) over time (Table 3).
Various TH methods were performed (Table 6). Several TH (external and internal cooling) methods were simultaneously used during the induction period and devices with feedback systems were used in 758 patients (81.5%). The manual passive rewarming method without a device was used in 39 patients (4.2%).
DISCUSSIONOf the total 930 registered OHCA survivors treated with TH, 556 (59.8%) survived to discharge and 249 (26.8%) were discharged with good neurological outcomes. The median time from ROSC to TH start was 101 minutes (IQR, 46 to 200 minutes), and the median rewarming duration was 708 minutes (IQR, 420 to 900 minutes). The time from ROSC to TH start decreased and rewarming duration increased annually. Coronary angiography was performed in 236 patients (25.4%); although the time to angiography decreased annually, it took longer to perform coronary angiography. The most frequent complications during TH were hyperglycemia, pneumonia, hypotension, seizure, and hypokalemia.
The two randomized controlled trials that proved the clinical effectiveness of TH enrolled only patients with OHCA and ventricular fibrillation [19,20]. Cardiac arrest survivors with a non-shockable rhythm had low proportion of good neurological outcomes and survival compared to patients with a shockable rhythm, and the AHA recommends class IIb TH in patients with OHCA and a non-shockable rhythm since its effectiveness in patients with OHCA and a non-shockable rhythm has not been proven [6,8,21,22]. Previous studies have analyzed the outcomes independent of the first monitored rhythm because the shockable rhythm was associated with good outcomes [8,21,22]. A study using a retrospective registry demonstrated that patients with OHCA and a shockable rhythm had a 56% chance of a good neurological outcome and a 61% chance of survival [21]. A study by Dumas et al. [8] reported that patients with OHCA and a shockable rhythm had a 44% chance of a good neurological outcome. A study by Soga et al. [22] also reported that patients with OHCA and a shockable rhythm had a 66% chance of good neurologic outcome at 30 days after cardiac arrest. The patients with OHCA and a shockable rhythm in the present study had a 60.5% chance of a good neurological outcome, similar to that observed in previous studies. Dumas et al. [8] reported that patients with OHCA and a non-shockable rhythm had a 15% chance of a good neurological outcome; the present study showed the same result (14.6%).
The optimal TH start time has not yet been identified. The Hypothermia after Cardiac Arrest (HACA) study group started TH within 105 minutes (IQR, 61 to 192 minutes) after ROSC [20]. In an animal study, the delayed start of TH attenuated its beneficial effect [23]. Therefore, an early TH start after ROSC is commonly accepted. Recent studies using a registry described TH start times after ROSC of 90 minutes (IQR, 60 to 165 minutes) and 57.5 minutes (IQR, 21 to 138 minutes), relatively earlier than that of the HACA study [13,21,23]. The TH start time in the present study was 101 minutes (IQR, 46 to 200 minutes). TH was thought to be performed more actively than before because it was started earlier; however, the TH start time was not associated with outcomes [21]. Since TH start time might not be a variable that influences clinical outcomes, other powerful factors must be considered.
Rapid induction to achieve the target temperature is recommended to avoid risks and side effects such as shivering and metabolic disorders [24]. The HACA study reported a median induction period of 8 hours (IQR, 4 to 16 hours).[20]. However, recently reported studies using registry data demonstrated shorter induction periods of 260 minutes (IQR, 178 to 400 minutes) and 3.0 hours (IQR, 1.3 to 5.8 hours) [13,21]. The median induction period in the present study was similar with those of previous studies using a registry.
The optimal maintenance period has not been identified either. The AHA currently recommends a maintenance period of 12–24 hours since the two randomized controlled trials, to date, maintained TH for 12 or 24 hours [6,19,20]. Several other studies have also reported a maintenance period of 12 to 24 hours [10,25-27]. A study using a registry performed in Japan reported a longer median maintenance period of 25 hours (IQR, 24 to 43 hours) and found no association between maintenance period and outcomes [13]. However, a well-controlled target temperature for >18 hours correlated with good neurological outcomes [11]. The quality of the maintenance period might be more critical than its duration.
Rewarming starts after completion of the maintenance period. Slow rewarming helps avoid side effects such as electrolyte imbalance, hypoglycemia, and hyperthermia [24]. The AHA has no detailed recommendation about the rewarming period, but several studies set a rewarming rate of 0.25°C/hr to 0.5°C/hr [6,8,9,12]. The median rewarming duration was 708 minutes (IQR, 420 to 900 minutes) in the present study and became adequate over time.
Coronary reperfusion therapy is one of the cornerstones of post-cardiac arrest care [6], while the effectiveness of coronary angiography following ROSC is controversial. However, coronary angiography can be performed with TH in cardiac arrest survivors [21,28]. Coronary angiography was performed in 41.8% of cardiac etiologies in the present study. However, most uses were not performed as post-cardiac arrest care since the procedure is time-intensive, although the time required improved over time.
A study that included 765 OHCA survivors from 22 facilities demonstrated that pneumonia (48%), hyperglycemia (37%), and seizure (24%) were frequent complications and found that hyperglycemia and the use of antiepileptic drugs were associated with mortality [29]. A meta-analysis study that analyzed 63 clinical studies including cardiac arrest survivors treated with TH reported that hyperglycemia (52.4%), pneumonia (38.0%), and hypotension (20.8%) were frequent complications [30]. Hyperglycemia (46.3%), pneumonia (37.1%), hypotension (36.0%), seizure (31.4%), and hypokalemia (27.4%) were frequent complications in the present study. Hyperglycemia and pneumonia were the most frequent complications seen here, similar with previous studies, although the definition of complications differed among the studies.
Our study has several limitations. First, we cannot demonstrate the ratio of TH application, total OHCA survival rate, and TH effectiveness since the KORHN registry included only patients with OHCA who were treated with TH. Second, selection bias could not be avoided because most of the included facilities were teaching or university-affiliated hospitals located within the nation’s capital region. Third, several data points were missing, which could affect the results, although the data manager and clinical research associates monitored the data and gave feedback to the principal investigators. Fourth, the KORHN registry was an emergency department-based registry, meaning that it could underestimate the number of patients transferred to a funeral home. This could be one of the reasons for the high survival rate reported here.
In summary, of the total 930 OHCA survivors, 59.8% survived to discharge and 26.8% were discharged with good neurological outcomes. The cooling start time and rewarming duration were appropriately managed according to current TH practice recommendations. Hyperglycemia and pneumonia were the most frequent complications.
REFERENCES1. Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation 2009; 80:297-305.
2. Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke 1989; 20:904-10.
3. Chopp M, Knight R, Tidwell CD, Helpern JA, Brown E, Welch KM. The metabolic effects of mild hypothermia on global cerebral ischemia and recirculation in the cat: comparison to normothermia and hyperthermia. J Cereb Blood Flow Metab 1989; 9:141-8.
4. Natale JA, D’Alecy LG. Protection from cerebral ischemia by brain cooling without reduced lactate accumulation in dogs. Stroke 1989; 20:770-7.
5. Sterz F, Leonov Y, Safar P, et al. Multifocal cerebral blood flow by Xe-CT and global cerebral metabolism after prolonged cardiac arrest in dogs: reperfusion with open-chest CPR or cardiopulmonary bypass. Resuscitation 1992; 24:27-47.
6. Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122(18 Suppl 3):S768-86.
7. Mikkelsen ME, Christie JD, Abella BS, et al. Use of therapeutic hypothermia after in-hospital cardiac arrest. Crit Care Med 2013; 41:1385-95.
8. Dumas F, Grimaldi D, Zuber B, et al. Is hypothermia after cardiac arrest effective in both shockable and nonshockable patients?: insights from a large registry. Circulation 2011; 123:877-86.
9. Lundbye JB, Rai M, Ramu B, et al. Therapeutic hypothermia is associated with improved neurologic outcome and survival in cardiac arrest survivors of non-shockable rhythms. Resuscitation 2012; 83:202-7.
10. Testori C, Sterz F, Behringer W, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation 2011; 82:1162-7.
11. Shinozaki K, Oda S, Sadahiro T, et al. Duration of well-controlled core temperature correlates with neurological outcome in patients with post-cardiac arrest syndrome. Am J Emerg Med 2012; 30:1838-44.
12. Kim JJ, Yang HJ, Lim YS, et al. Effectiveness of each target body temperature during therapeutic hypothermia after cardiac arrest. Am J Emerg Med 2011; 29:148-54.
13. Yokoyama H, Nagao K, Hase M, et al. Impact of therapeutic hypothermia in the treatment of patients with out-of-hospital cardiac arrest from the J-PULSE-HYPO study registry. Circ J 2011; 75:1063-70.
14. Italian Cooling Experience (ICE) Study Group. Early- versus late-initiation of therapeutic hypothermia after cardiac arrest: preliminary observations from the experience of 17 Italian intensive care units. Resuscitation 2012; 83:823-8.
15. Wolfrum S, Radke PW, Pischon T, Willich SN, Schunkert H, Kurowski V. Mild therapeutic hypothermia after cardiac arrest: a nationwide survey on the implementation of the ILCOR guidelines in German intensive care units. Resuscitation 2007; 72:207-13.
16. Bouwes A, Kuiper MA, Hijdra A, Horn J. Induced hypothermia and determination of neurological outcome after CPR in ICUs in the Netherlands: results of a survey. Resuscitation 2010; 81:393-7.
17. Kim JY, Shin SD, Ro YS, et al. Post-resuscitation care and outcomes of out-of-hospital cardiac arrest: a nationwide propensity score-matching analysis. Resuscitation 2013; 84:1068-77.
18. Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004; 291:870-9.
19. Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557-63.
20. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549-56.
21. Nielsen N, Hovdenes J, Nilsson F, et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand 2009; 53:926-34.
22. Soga T, Nagao K, Sawano H, et al. Neurological benefit of therapeutic hypothermia following return of spontaneous circulation for out-of-hospital non-shockable cardiac arrest. Circ J 2012; 76:2579-85.
23. Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993; 21:1348-58.
24. Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009; 37:1101-20.
25. Busch M, Soreide E, Lossius HM, Lexow K, Dickstein K. Rapid implementation of therapeutic hypothermia in comatose out-of-hospital cardiac arrest survivors. Acta Anaesthesiol Scand 2006; 50:1277-83.
26. Kliegel A, Janata A, Wandaller C, et al. Cold infusions alone are effective for induction of therapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation 2007; 73:46-53.
27. Merchant RM, Abella BS, Peberdy MA, et al. Therapeutic hypothermia after cardiac arrest: unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med 2006; 34:S490-4.
28. Reynolds JC, Callaway CW, El Khoudary SR, Moore CG, Alvarez RJ, Rittenberger JC. Coronary angiography predicts improved outcome following cardiac arrest: propensity-adjusted analysis. J Intensive Care Med 2009; 24:179-86.
Fig. 2.Survival and neurological outcomes at discharge. Survival and neurological outcomes at discharge of the 930 patients with out-of-hospital cardiac arrests (OHCAs) treated with therapeutic hypothermia (TH) included in the study, divided into first monitored rhythm of ventricular fibrillation (Vf)/pulseless ventricular tachycardia (VT), asystole, pulseless electrical activity (PEA), and unknown. A good neurological outcome was defined as cerebral performance category (CPC) 1 or 2. 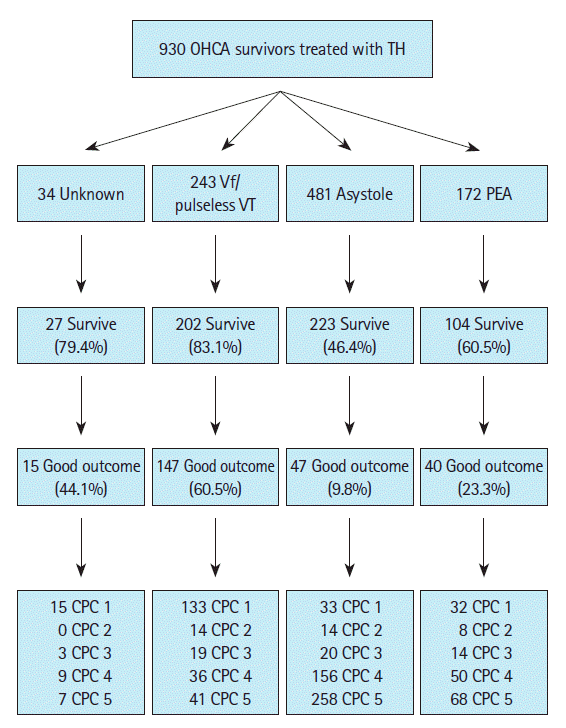
Table 1.Demographic data of the patients and cardiac arrest events
Table 2.Therapeutic hypothermia characteristics
Table 3.Performance of therapeutic hypothermia and coronary angiography after return of spontaneous circulation by year Table 4.Adverse events during therapeutic hypothermia Table 5.Coronary reperfusion and other circulatory supportive therapies (n=930)
Table 6.Therapeutic hypothermia methods |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||