INTRODUCTION
Atraumatic pericardial tamponade and intracardiac masses are both recognized etiologies of acute obstructive shock. Pericardial tamponade, is a cardiovascular emergency commonly considered by emergency physicians and, as a result, evaluation for this process has been incorporated into standardized point of care ultrasound (POCUS) algorithms for assessing hypotension [1]. Obstructive shock secondary to intracardiac tumors is an extremely rare clinical presentation and, although it is evaluated by the same ultrasound imaging modality, it is rarely considered, or evaluated for, in the emergency department (ED) setting. Furthermore, due to the extremely uncommon concomitant incidence of these two pathologic processes, cardiac tamponade as the initial manifestation of an intracardiac tumor has almost no literary precedence in the emergency medicine literature despite its unique clinical features and significant clinical implications.
CASE REPORT
A 24-year-old female patient with no past medical history presented to the ED with 1 week of intermittent abdominal pain. The patient reported no fever, and prior to arrival in the ED had one episode of nonbilious and nonbloody vomiting. The patient reported no chest pain, shortness of breath, hemoptysis or any other cardiopulmonary symptomatology, as well as no dysuria or other urinary complaints.
In the ED, the patientŌĆÖs initial vital signs were temperature 36.8┬░C, blood pressure 123/86 mmHg, heart rate 124 beats/min, and oxygen saturation 100% on room air. The patientŌĆÖs general appearance was significant for perioral and distal upper extremity cyanosis. There were no muffled heart sounds, rubs, gallops, or murmurs on cardiac exam. Lungs were clear to auscultation bilaterally. The abdomen was soft with mild epigastric and right upper quadrant tenderness. No rebound or guarding were noted. There was no lower extremity edema. Initial laboratory studies included a normal complete blood count with no evidence of anemia, neutropenia or thrombocytopenia. Complete metabolic and hepatobiliary panels were significant for bilirubin 1.5 mg/dL, alanine aminotransferase/aspartate aminotransferase 49/43 U/L, and lactate 3.0 mmol/L. The patient was noted to have a beta Human Chorionic Gonadotropin (HCG) of 0 IU/L.
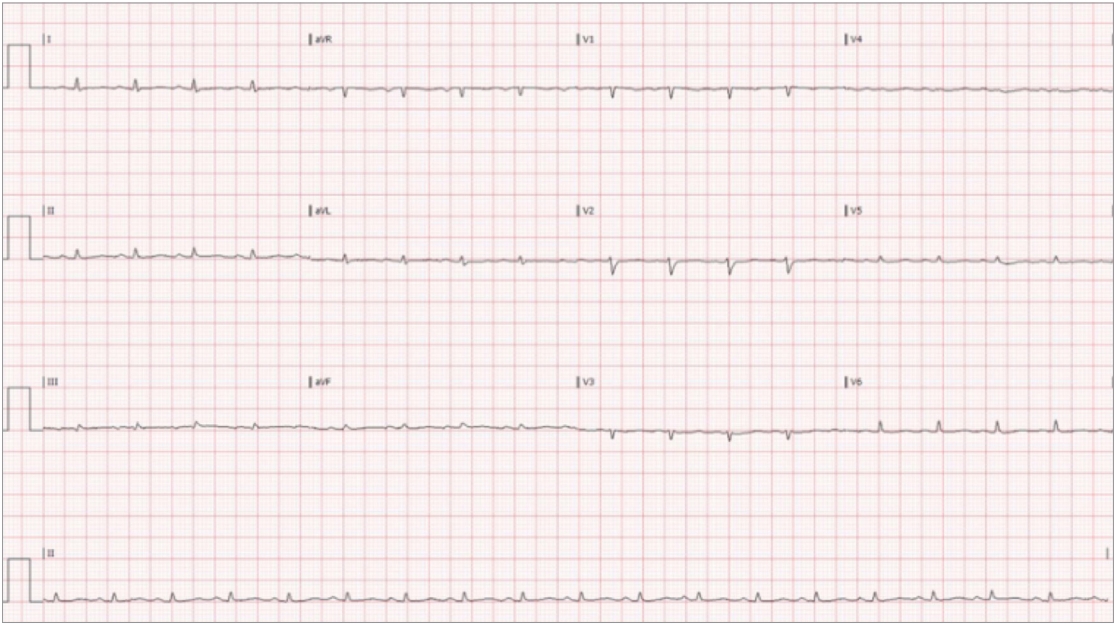
Due to the primary complaint of abdominal pain the patient was taken for computed tomography imaging which showed findings concerning for a metastatic disease process, including multiple liver lesions as well as evidence of pleural and pericardial fluid accumulation. Upon returning to the ED, approximately 6 hours of initial presentation to the ED, the patient was noted to have markedly worsening dyspnea, cyanosis and jugular venous distention. Persistent tachycardia was also appreciated with an electrocardiogram showing sinus tachycardia with low voltage (Fig. 1). Emergent evaluation with cardiac windows of the rapid ultrasound for shock and hypotension (RUSH) exam, via a phased array probe, demonstrated a large pericardial effusion. Swinging of the heart in the accumulated fluid as well as right ventricular collapse during diastole were seen on the ED POCUS exam, consistent with radiographic findings of shock secondary to tamponade. The patient subsequently developed hypotension with a blood pressure of 90/60 mmHg. Other pertinent findings in the ED included a chest X-ray consistent with the large pericardial effusion and "water-bottle" heart (Fig. 2).
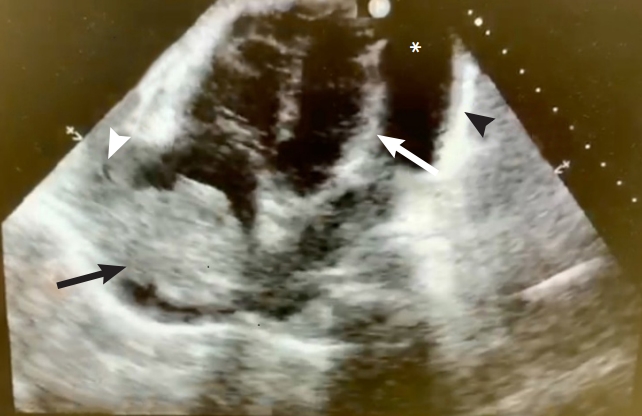
The patient was admitted to the cardiac intensive care unit where additional ultrasound imaging demonstrated a large 5├Ś5-cm space occupying mass in the posterior wall of the right atrium with extension into the superior vena cava (Fig. 3). The patient underwent an emergent pericardiocentesis with the removal of 2 L of serosanguinous fluid only partially improving the patientŌĆÖs shock. A drain was left in place. However, despite definitive management anticipated improvement in blood pressure was not achieved and the patient remained hypotensive. These findings were thought to be secondary to the intracardiac obstructing mass. A repeat electrocardiogram demonstrated persistently low voltage but with a marked improvement of the patientŌĆÖs heart rate (Fig. 4). Subsequently, the patient was transferred to another center with extracorporeal membrane oxygenation capabilities, however, this therapy was never initiated. Cytology of the pericardial fluid demonstrated that the accumulating effusion contained no malignancy. Subsequent biopsy studies of the mass were consistent with a primary cardiac angiosarcoma. The patient underwent chemotherapy followed by surgical debulking with clinical improvement. The patient provided written informed consent for publication of the research details and clinical images.
DISCUSSION
Atraumatic pericardial effusion, with and without tamponade physiology, is a pathologic process encountered by emergency physicians, reflecting a wide differential diagnosis [2]. In their most extreme physiologic manifestation large, or rapidly accumulating, effusions can lead to cardiac tamponade, extravascular obstructive shock and cardiovascular collapse. It has been reported that 11% of all pericardial effusions test positive for malignancy. However, the rate of pericardial effusions secondary to an underlying malignancy, without findings of malignant cells via cytology is not clearly established [3]. Our case, however, is consistent with the prior literature, where effusions secondary to cardiac angiosarcomas, and other primary cardiac tumors, do not demonstrate malignant cells during cytological examination [4,5]. The underlying physiologic mechanism for cardiovascular collapse is based on impaired left ventricular filling (preload) secondary to external pressure on the myocardium resulting in limited ventricular relaxation during diastole. This ultimately results in diminished cardiac output, systemic hypoperfusion and shock [6].
The commonly accepted practice of assessing for pericardial effusions and tamponade in the ED for patients presenting with both cardiovascular symptomatology and hemodynamic instability has led to the nearly ubiquitous use of the POCUS modality with established imaging protocols in the form of the FAST (focused assessment with sonography in trauma) and RUSH examination, which both include primary cardiac views [1]. Historically, early clinical signs of acute cardiopulmonary compromise- including unexplained tachycardia, cyanosis and jugular venous distention, all features consistent with our patientŌĆÖs presentation- should prompt further investigation with point of care cardiac imaging. For nearly a decade the use of POCUS examinations for the assessment of cardiovascular shock, has been formally accepted in the emergency medicine community by its governing academic bodies [7]. In addition, extensive clinical research has demonstrated the efficacy of this modality when used by emergency medicine physicians [8]. Furthermore, it has been well established that most clinical findings, including diminished heart sounds which were not appreciated in our patient, have extremely poor sensitivity limiting their contributions to diagnosis and management [9].
Primary cardiac tumors are extremely rare with a reported prevalence of 0.0001% to 0.003% [10]. Cardiac angiosarcomas commonly originate from vascular endothelial cells. They typically occur in the right atrium, are often advanced on initial presentation, and may compromise blood flow from the vena cava to the right atrium leading to an intravascular obstructive shock process with hemodynamic compromise. Specifically, cardiac masses located at the cavoatrial junction, as well as those abutting intracardiac valves, have been known to obstruct left ventricular filling and ultimately cardiac output leading to a true, but often underrecognized, oncologic emergency [11]. While POCUS ultrasonography is not currently a definitive study in assessing for intracardiac masses, abnormal findings should prompt further evaluation via comprehensive echocardiography or another imaging modality.
In contrast to pericardial tamponade, a clinical process regularly considered and evaluated within the ED, intracardiac tumors, which can be assessed via the same imaging modality, are rarely ever considered as an underlying etiology of acute shock. It is, however, well established that goal directed ultrasound protocols in nontraumatic, symptomatic hypotensive patients can improve diagnostic accuracy in the undifferentiated patient [12]. To date, there are limited existing reports dealing with presentation of cardiac angiosarcomas with associated pericardial tamponade, and virtually no prior references in the emergency medicine literature. In some instances, clinicians may overlook primary cardiac masses as the underlying cause of a pericardial effusion [13]. The limited case reports that do exist suggest that the development of pericardial effusions are possible secondary to compromise of integrity of the atrial wall [14,15]. Nearly none of the previous literature discuss the relationship between both intravascular and extravascular obstructive shock secondary to a concomitant tamponade and intracardiac obstructive physiology.
Ultimately, the copresentation of these two pathologic processes is extremely rare. Existing literature is found in a small number of case reports with nearly no prior descriptions in emergency medicine references. In the correct clinical context this unique presentation should be considered and evaluated for in the ED via POCUS modality to help guide in the management of resulting obstructive shock.