AbstractObjectiveWe evaluated the utility of the Korean Modified Early Warning Score (KMEWS), which combines the Modified Early Warning Score (MEWS) and the Korean Triage and Acuity Scale (KTAS), as a triage tool to screen for infection in patients who visit the emergency department.
MethodsWe retrospectively reviewed data extracted from electronic medical records. Patients aged ≥18 years with an infection who were admitted to the hospital via the emergency department between January 2018 and December 2019 were eligible for inclusion. The KMEWS score was calculated as the sum of the KTAS level and the MEWS score. We generated receiver operating characteristic curves and determined the area under the receiver operating characteristic curve (AUC) for the KMEWS, KTAS, MEWS, and Mortality in Emergency Department Sepsis (MEDS) scales. The primary outcome was septic shock, and secondary outcomes were intensive care unit admission and in-hospital mortality.
ResultsThe AUC values (95% confidence interval) for predicting septic shock were as follows: KMEWS, 0.910 (0.902–0.918); MEWS, 0.896 (0.887–0.904); KTAS score, 0.809 (0.798–0.819); and MEDS, 0.927 (0.919–0.934). The AUC values (95% confidence interval) for predicting in-hospital mortality were as follows: KMEWS, 0.752 (0.740–0.764); MEWS, 0.717 (0.704–0.729); KTAS score, 0.764 (0.752–0.776); and MEDS, 0.844 (0.834–0.854). The AUC values (95% confidence interval) for predicting intensive care unit admission were as follows: KMEWS, 0.826 (0.816–0.837); MEWS, 0.782 (0.770–0.793); KTAS score, 0.821 (0.810–0.831); and MEDS, 0.839 (0.829–0.849).
INTRODUCTIONSepsis is an inflammatory disease caused by a reaction of the immune system, which can be life-threatening and is responsible for 20% of all hospital deaths each year [1,2]. In patients with sepsis, early resuscitation and intensive care are associated with a lower mortality rate compared with later resuscitation [3]. Therefore, the recognition of sepsis and the timely initiation of evidence-based protocols are critical [4,5]. However, the diagnosis of sepsis using the Sequential Organ Failure Assessment (SOFA) score is time-consuming in overcrowded emergency departments (EDs) [6]. Several markers for predicting septic shock are known, but most of them require laboratory tests that require time. Therefore, a new tool is required that can predict septic shock early in the triage stage [7-11].
The Modified Early Warning Score (MEWS) is a simple physiological score used to screen patients at risk of clinical deterioration using body temperature, blood pressure, pulse rate, respiratory rate, and level of consciousness values and to allow for the early detection of clinical deterioration and the potential need for a higher level of care (Supplementary Table 1) [12,13]. In Korea, the Korean Triage and Acuity Scale (KTAS) was developed based on the Canadian Triage and Acuity Scale and has been used since 2016. The KTAS is a combination of variables, including vital signs and chief complaints, and serves as a tool for determining the severity of the patients’ condition and the priority for treatment in the ED (Supplementary Table 2) [14,15]. The two scoring systems might have different strengths and weaknesses depending on whether the chief complaints are included and whether the hemodynamic variables are subdivided. Therefore, this study was conducted to investigate whether the Korean Modified Early Warning Score (KMEWS), which combines the MEWS and the KTAS scores, is useful as a triage tool to screen patients with infection in the ED.
METHODSEthical statementsThe study was approved by the Institutional Review Board of Chungnam National University Hospital (No. 2020-10-059). The need for informed consent was waived because of the retrospective study design and the use of anonymized data. Only clinical data were extracted, and no personal or identifiable information was recorded.
Study design and settingWe retrospectively reviewed data extracted from electronic medical records. The study sample included patients aged ≥18 years with infections who were admitted to the hospital via the ED between January 2018 and December 2019 at a tertiary care university hospital with 1,350 beds in Daejeon, Korea. The ED provides medical care to approximately 55,000 patients per year. Patients with missing data were excluded.
The diagnosis of infection was confirmed using the relevant International Classification of Diseases, 10th Revision (ICD-10) codes in the medical records. Patients with any of the following infection-related ICD-10 codes were eligible for enrollment: A00–B99, G00–09, I00–02, I30–33, I38–41, J00–22, J36, J37, J40–J43, J68, J69, J80, J85–J86, K11–12, K35–37, K57, K61, K63, K65, K67, K75, K77.0, K80–81, K83.0, K85, L00–08, M00–03, M86, N10, N12, N13.6, N16.0, N28.84–28.86, N30, N34, N39.0, N41, N45, N61, N70–74, and O91.
Sepsis patients were defined by the presence of two or three of the three quick SOFA (qSOFA) clinical criteria (altered mentation, respiratory rate ≥22 breaths/min, and systolic blood pressure ≤100 mmHg) [16]. Among patients with sepsis, septic shock was clinically defined as a case where a vasopressor was required to maintain a mean arterial pressure of ≥65 mmHg and a serum lactate level >2 mmol/L (>18 mg/dL) in the ED or during hospitalization [16].
Data collection and outcome measuresWe collected clinical data from the patients’ electronic medical records. The information included age, sex, systolic arterial pressure (mmHg), respiratory rate (breaths/min), body temperature (°C), and mental status. We calculated the Charlson Comorbidity Index, which categorizes the comorbidities of patients based on the ICD diagnosis codes found in the administrative data [17], for each patient. In addition, we calculated the Mortality in Emergency Department Sepsis (MEDS) score, the MEWS score, and the KTAS score [7-9,12,13]. The numbers of points on the KTAS score were 5, 4, 3, 2, or 1 for levels 1, 2, 3, 4, and 5, respectively. The KMEWS was calculated as the sum of the KTAS score and the MEWS score. Since both KTAS and MEWS have initial vital signs, the problem of vital signs over weighting is raised. thus obtained KMEWS-2 by combining KTAS with the score subtracting the initial vital signs from MEWS. In addition, we divided the participants into septic shock and nonseptic shock groups and conducted intergroup comparisons. The primary outcome of this study was septic shock, and secondary outcomes were intensive care unit (ICU) admission and in-hospital mortality.
Data analysisContinuous variables are expressed by mean±standard deviation or median (interquartile range, IQR). Continuous variables were analyzed using the Student t-test or the Mann-Whitney U-test, and categorical variables were analyzed using chi-square or Fisher exact tests. A multivariable logistic regression was performed to identify predictive factors for septic shock using variables that had previously been reported to be significantly associated with septic shock.
We generated receiver operating characteristic (ROC) curves and determined the area under the ROC curve (AUC) for individual measures (KMEWS, KTAS, MEWS, and MEDS) that was associated with septic shock, ICU admission, and in-hospital mortality. The AUCs of the models were calculated and tested mutually for significance using DeLong equality tests. In addition, the cutoff value was calculated using the Youden index (Youden’s J statistic). All statistical analyses were performed using the IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA) and MedCalc ver. 14.8.1 (MedCalc software, Ostend, Belgium). P-values less than 0.05 were considered statistically significant.
RESULTSPatient characteristicsWe enrolled 19,228 patients during the study period, of which 7,907 patients had infection-related diagnosis at discharge, and 2,814 patients with missing data were excluded. If data such as blood test items, history, and state of consciousness were collected inadequately, they were treated as missing data. Thus, 5,093 patients of these were included in the analysis data set (Fig. 1). Among them, 395 and 4,698 patients were in the septic shock and nonseptic shock groups, respectively.
A comparison of the characteristics of the septic shock group and the nonseptic shock group
Table 1 shows a comparative analysis of two groups, patients without septic shock and patients with septic shock, and their baseline characteristics, laboratory findings, and several infection-related markers. Intergroup comparisons of the septic shock group and the nonseptic shock group showed no significant difference in sex; however, the participants in the septic shock group were older (78 years [IQR, 68–83 years] vs. 61 years [42.0–76.0 years], P<0.001). In the septic shock group, the systolic arterial pressure was lower than that in the nonseptic shock group (99 mmHg [IQR, 85–127 mmHg] vs. 125 mmHg [111–140 mmHg], P<0.001), and a higher proportion of patients had an altered mental status (72.4% vs. 6.0%, P<0.001). The septic shock group had a higher Charlson Comorbidity Index (5 [IQR, 4–6] vs. 2 [IQR, 0–5], P<0.001). The laboratory results were generally worse in the septic shock group (Table 1). The septic shock group had a significantly higher proportion of patients with KTAS levels 1 or 2 (7.3% vs. 56.1%, P<0.001) and higher MEWS, KTAS, and KMEWS scores (Table 1).
Variables associated with septic shock upon multivariable logistic regressionA multivariable logistic regression revealed that age, transfer from a long-term care facility, KTAS score, and MEWS score were significantly associated with septic shock (Table 2). The adjusted odds ratio was 2.18 (95% confidence interval [CI], 1.76–2.70) for KTAS and 2.09 (95% CI, 1.93–2.27) for MEWS.
ROC curve analysisIn the ROC analysis, the AUC values (95% CI) of factors associated with septic shock were as follows: KMEWS, 0.910 (0.902–0.918); MEWS, 0.896 (0.887–0.904); KTAS score, 0.809 (0.798–0.819); and MEDS score, 0.927 (0.919–0.934) (Fig. 2). The optimal cutoff value of the KMEWS for predicting septic shock was 7 (AUC, 0.910; sensitivity, 81.3% [95% CI, 77.1%–85.0%]; specificity, 82.6% [95% CI, 81.5%–83.7%]). The respective AUC values (95% CI) of the factors associated with mortality were KMEWS, 0.752 (0.740–0.764); MEWS, 0.717 (0.704–0.729); KTAS score, 0.764 (0.752–0.776); and MEDS, 0.844 (0.834–0.854) (Fig. 3). The respective AUC values (95% CI) of factors associated with ICU admission were as follows: KMEWS, 0.826 (0.816–0.837); MEWS, 0.782 (0.770–0.793); KTAS score, 0.821 (0.810–0.831); and MEDS, 0.839 (0.829–0.849) (Fig. 4). In addition, the respective AUC values (95% CI) of factors associated with septic shock were as follows: KMEWS, 0.910 (0.902–0.918); MEWS, 0.896 (0.887–0.904); KTAS score, 0.809 (0.798–0.819); MEDS, 0.927 (0.919–0.934); and KMEWS-2, 0.894 (0.885–0.902) (Supplementary Fig. 1).
DISCUSSIONIn this study, the KMEWS, a combination of the MEWS and KTAS scores, was a useful prognostic marker for patients with infection, particularly for predicting septic shock. In the ED, triage helps predict the severity of conditions and determines the priority of patient treatment [18]. The most well-known triage scales are the Emergency Severity Index in the United States, the Canadian Triage and Acuity Scale in Canada, the Australian Triage Scale in Australia, and the Manchester Triage Scale in the United Kingdom [18,19]. In Korea, the KTAS was developed based on the Canadian Triage and Acuity Scale and was implemented in 2015. The KTAS consists of a five-level system that classifies patients using a combination of variables, including vital signs and chief complaints [14,15]. Since the implementation of the KTAS, the admission and disposition patterns have changed and resulted in reduced mortality in the ED [14].
Patients visit the ED with a wide variety of complaints, but the proportion of patients with infection is substantial [20,21]. Because the early diagnosis of sepsis or septic shock is one of the most important factors that affects the success of treatment, many scoring systems that use various markers have been used [22,23]. However, the current triage tools are inadequate for determining the severity and prognosis of patients presenting to the ED with infection [24,25].
The qSOFA score is an established screening tool for sepsis [6]. However, the qSOFA is not suitable as a screening tool because of its low sensitivity [26]. The MEDS score consists of nine factors that are associated with a greater mortality risk, including an age >65 years, an altered mental status, and a terminal illness. The MEDS score has moderate accuracy in predicting mortality in ED patients with suspected infection, and the MEDS is superior to the MEWS in predicting mortality in this patient population [7-9]. However, the nine factors that comprise the MEDS score include the platelet count and neutrophil count; since it takes time to obtain the score, it is thus not suitable for use as a triage screening tool in the ED.
The Early Warning Score is a simple physiological scoring system that can be easily applied at the bedside [12]. The MEWS is used as a screening tool for septic shock patients who are at risk of clinical deterioration using the values of temperature, blood pressure, pulse, respiratory rate, and level of consciousness. The MEWS may be useful for screening patients with septic shock [12,13,16,27]. Moreover, the MEWS does not require laboratory test results; therefore, it is immediately available during triage.
However, as the MEWS is somewhat nonspecific and does not contain factors related to the chief complaint, there is a limitation in its use for patients with infection. In contrast, the KTAS includes the chief complaint and the vital signs, but the hemodynamic criteria are not subdivided. The KTAS and MEWS can be applied to ED triage because laboratory results are not required. Therefore, we hypothesized that supplementing the physiological data with the MEWS and KTAS scores could help determine a prompt prognosis in infected patients.
As the KTAS includes both vital signs and chief complaints, and the MEWS includes vital signs, the KMEWS (the sum of the KTAS and the MEWS scores) has a weighting value for the initial vital signs. In the multivariable logistic regression analysis of this study, the KTAS and KMEWS scores were independently associated with septic shock and showed similar odds ratios for septic shock. Therefore, the KMEWS was calculated by combining the two scores. As a result, the KMEWS showed similar or higher AUC values for septic shock, ICU admission, and mortality compared to either the KTAS score or the MEWS score alone. The MEDS had a slightly higher AUC value than the KMEWS, but it is unsuitable for use as a septic shock screening tool in the ED. Therefore, the KMEWS could be a useful prognostic tool for triaging patients with septic shock in the ED.
Nevertheless, this study had some limitations. First, it was a single-center observational study that included only patients admitted to the hospital via the ED. Patients who had been transferred from another hospital or who died in the ED were excluded. Therefore, the generalizability of our results may be limited. Second, it included a collection of retrospective data that could introduce potential information biases and contained much missing data. Third, the sepsis diagnosis process was excluded because it was a retrospective study of patients who had already been diagnosed with an infectious disease. Inclusion criteria in our study were based on ICD-10 codes related to infection, and no blood culture reports were available. The diagnosis of septic shock was defined as sepsis with a serum lactate level >2 mmol/L, which did not reflect the patient’s volume status. Finally, there could have been inter-clinician variability in calculating the KTAS score and the MEWS score during triage.
The KMEWS, which is a combination of the MEWS and the KTAS scores, could be a useful triage tool for screening patients for septic shock in the ED. In addition, it showed acceptable predictive power for mortality or ICU admission in patients with infection. Prospective multicenter studies are necessary to validate these findings.
SUPPLEMENTARY MATERIALSupplementary materials are available at https://doi.org/10.15441/ceem.22.339.
Supplementary Table 1.Calculation of the Modified Early Warning Score
Supplementary Table 2.Definitions, related conditions, and corresponding medical actions of the Korean Triage and Acuity Scale
Supplementary Fig. 1.Analysis of receiver operating characteristics curves for predicting septic shock.
NOTESAUTHOR CONTRIBUTIONS
Conceptualization: SR; Data curation: SR, SKO; Formal analysis: SKO, BKL; Funding acquisition: SKO; Investigation: SKO, BKL; Methodology: SR, SJ; Project administration: SR, SJ; Resources: SR, SKO; Software: SR, SJ; Supervision: SR, SKO; Validation: SR, SKO; Visualization: SR, BKL; Writing–original draft: SR, SKO, SJ; Writing–review & editing: all authors.
All authors read and approved the final manuscript.
REFERENCES1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303-10.
2. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med 2006; 34:15-21.
3. Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med 2008; 36:2734-9.
4. Gauer RL. Early recognition and management of sepsis in adults: the first six hours. Am Fam Physician 2013; 88:44-53.
5. Ferrer R, Artigas A, Suarez D, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med 2009; 180:861-6.
6. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:762-74.
7. Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in emergency department sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med 2003; 31:670-5.
8. Chang SH, Hsieh CH, Weng YM, et al. Performance assessment of the Mortality in Emergency Department Sepsis Score, Modified Early Warning Score, Rapid Emergency Medicine Score, and Rapid Acute Physiology Score in predicting survival outcomes of adult renal abscess patients in the emergency department. Biomed Res Int 2018; 2018:6983568.
9. Zhang G, Zhang K, Zheng X, Cui W, Hong Y, Zhang Z. Performance of the MEDS score in predicting mortality among emergency department patients with a suspected infection: a meta-analysis. Emerg Med J 2020; 37:232-9.
10. Gunes Ozaydin M, Guneysel O, Saridogan F, Ozaydin V. Are scoring systems sufficient for predicting mortality due to sepsis in the emergency department? Turk J Emerg Med 2016; 17:25-8.
11. Kim SJ, Hwang SO, Kim YW, Lee JH, Cha KC. Procalcitonin as a diagnostic marker for sepsis/septic shock in the emergency department; a study based on Sepsis-3 definition. Am J Emerg Med 2019; 37:272-6.
12. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified early warning score in medical admissions. QJM 2001; 94:521-6.
13. Tavares RC, Vieira AS, Uchoa LV, Peixoto Junior AA, Meneses FA. Validation of an early warning score in pre-intensive care unit. Rev Bras Ter Intensiva 2008; 20:124-7.
14. Kwon H, Kim YJ, Jo YH, et al. The Korean Triage and Acuity Scale: associations with admission, disposition, mortality and length of stay in the emergency department. Int J Qual Health Care 2019; 31:449-55.
15. Park J, Lim T. Korean Triage and Acuity Scale (KTAS). J Korean Soc Emerg Med 2017; 28:547-51.
16. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801-10.
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373-83.
18. Christ M, Grossmann F, Winter D, Bingisser R, Platz E. Modern triage in the emergency department. Dtsch Arztebl Int 2010; 107:892-8.
19. Lahdet EF, Suserud BO, Jonsson A, Lundberg L. Analysis of triage worldwide. Emerg Nurse 2009; 17:16-9.
20. Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007; 35:1928-36.
21. ProCESS Investigators; Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683-93.
22. Fan SL, Miller NS, Lee J, Remick DG. Diagnosing sepsis: the role of laboratory medicine. Clin Chim Acta 2016; 460:203-10.
23. McLymont N, Glover GW. Scoring systems for the characterization of sepsis and associated outcomes. Ann Transl Med 2016; 4:527.
24. Jones AE, Shapiro NI, Roshon M. Implementing early goal-directed therapy in the emergency setting: the challenges and experiences of translating research innovations into clinical reality in academic and community settings. Acad Emerg Med 2007; 14:1072-8.
25. Carlbom DJ, Rubenfeld GD. Barriers to implementing protocol-based sepsis resuscitation in the emergency department: results of a national survey. Crit Care Med 2007; 35:2525-32.
Fig. 1.A flowchart of the study. Infection-related diagnosis was confirmed using the relevant International Classification of Diseases, 10th Revision codes in the medical records. ED, emergency department. 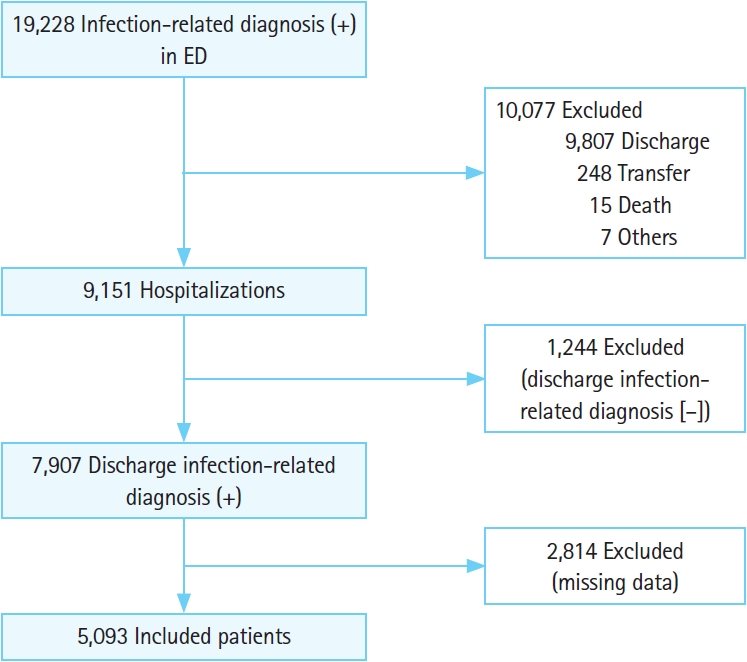
Fig. 2.An analysis of the receiver operating characteristic curves for predicting septic shock. The areas under the receiver operating characteristic curve (AUC) of the models were calculated and tested mutually for significance using DeLong equality tests. The Korean Modified Early Warning Score (KMEWS) is the sum of the Korean Triage and Acuity Scale (KTAS) score and the Modified Early Warning Score (MEWS) score. MEDS, Mortality in Emergency Department Sepsis; CI, confidence interval. 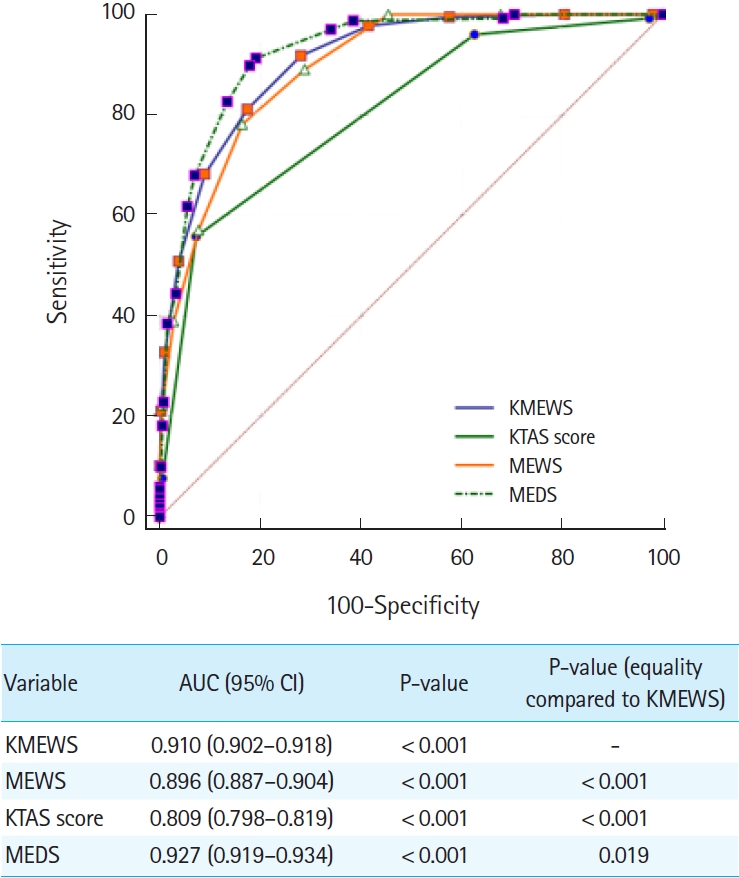
Fig. 3.An analysis of the receiver operating characteristic curves for predicting in-hospital mortality. The areas under the receiver operating characteristic curve (AUC) of the models were calculated and tested mutually for significance by DeLong equality tests. The Korean Modified Early Warning Score (KMEWS) is the sum of the Korean Triage and Acuity Scale (KTAS) score and the Modified Early Warning Score (MEWS) score. MEDS, Mortality in Emergency Department Sepsis; CI, confidence interval. 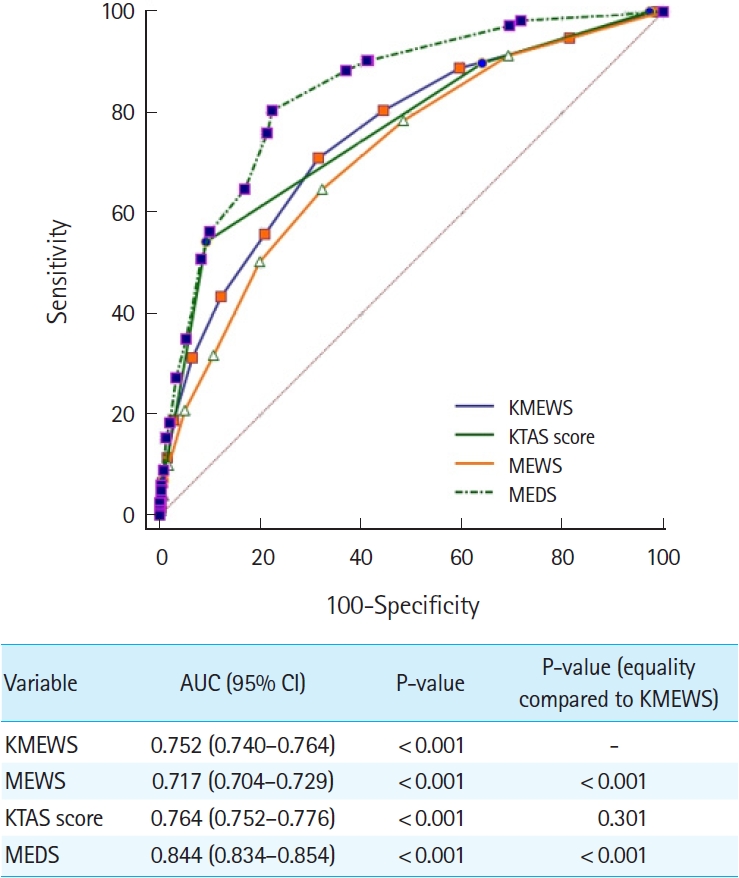
Fig. 4.An analysis of the receiver operating characteristics curves for predicting intensive care unit admission. The areas under the receiver operating characteristic curve (AUC) of the models were calculated and tested mutually for significance using DeLong equality tests. The Korean Modified Early Warning Score (KMEWS) is the sum of the Korean Triage and Acuity Scale (KTAS) score and the Modified Early Warning Score (MEWS) score. MEDS, Mortality in Emergency Department Sepsis; CI, confidence interval. 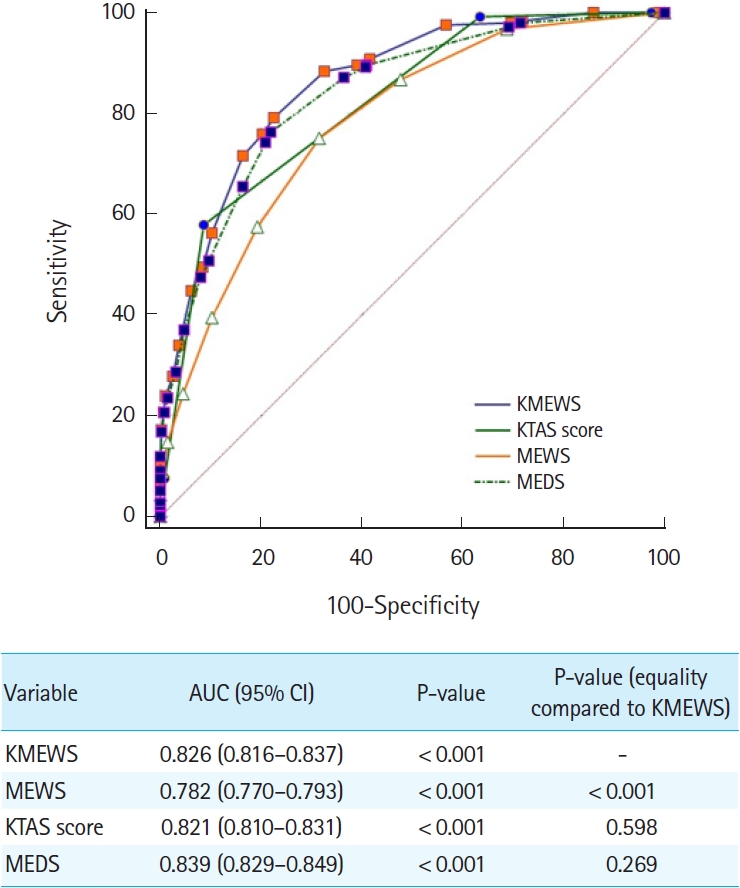
Table 1.The baseline characteristics of the study patients
Values are presented as median (interquartile range) or mean±standard deviation for continuous variables and number (%) for categorical variables. MEDS, Mortality in Emergency Department Sepsis; MEWS, Modified Early Warning Score; KTAS, Korean Triage Acuity Scale; KMEWS, Korean Modified Early Warning Score. Table 2.A multivariable logistic regression analysis of the factors associated with septic shock in patients in the emergency department |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||