AbstractThe novel SARS-CoV-2 emerged in 2019, and the global COVID-19 pandemic continues into 2022. It has been known that a subset of patients develops chronic, debilitating symptoms after otherwise complete recovery from acute infection of COVID-19. Multiple terms have been used to describe this constellation of symptoms, including long COVID, long-haul COVID, and postacute sequelae of SARS-CoV-2 syndrome (PASC). PASC is broadly defined as a wide range of new, returning, or ongoing symptoms at least four weeks after infection. Those patients are often seen in emergency departments after acute COVID-19 infection, but their symptoms are not adequately managed because the underlying pathophysiology of PASC is not well understood. Among patients with PASC, postural orthostatic tachycardic syndrome (POTS) has been increasingly recognized. POTS is one of the most common forms of autonomic dysfunction and defined by a sustained orthostatic tachycardia during active standing or head-up tilt test in the absence of orthostatic hypotension or other cardiopulmonary diseases. Because POTS is a treatable condition, it is important to recognize POTS among PASC patients. Herein, we reviewed the current literature on POTS and dysautonomia in PASC in order to better understand the overlap and distinction between these pathologies.
INTRODUCTIONThe novel SARS-CoV-2 emerged in 2019, and the global COVID-19 pandemic continues into 2022. Since the introduction of vaccination against COVID-19, the overall mortality has been declining [1–3]. However, a subset of patients develop chronic, debilitating symptoms after complete recovery from acute COVID-19 infection. Several terms have been used to describe this constellation of symptoms, including long COVID, long-haul COVID, and post-acute sequelae of SARS-CoV-2 (PASC) syndrome. PASC is broadly defined as a wide range of new, returning, or ongoing symptoms at least 4 weeks after infection [4]. Typical symptoms include chronic fatigue, chronic nausea, indigestion, constipation, brain fog, orthostatic tachycardia, and exertional dyspnea among many others. Symptoms are often severe, and most patients are on disability or modified independence. Due to the severity of the symptoms, these patients are often seen in emergency departments (EDs) after acute COVID-19 infection. However, the pathophysiology and treatments for PASC are not well established.
Although PASC has multiple manifestations, postural orthostatic tachycardia syndrome (POTS) has been increasingly recognized among patients with PASC. The diagnostic definition of POTS includes symptoms that have lasted longer than three months and a sustained heart rate increase of at least 30 beats/min or a heart rate of 120 beats/min or more within 10 minutes of standing or during head-up tilt test (HUTT) in the absence of orthostatic hypotension (OH) while reproducing typical symptoms [5]. The symptoms of POTS often include dizziness, palpitations, fatigue, headache, nausea, pre-syncope, tunneling of vision, and brain fog.
Many POTS symptoms are thought to be due to autonomic vasomotor failure. Because viral infection can host various chronic neurological conditions, such as Guillain-Barre syndrome, POTS can possibly occur as a post-COVID-19 autonomic nerve involvement. However, the prevalence of POTS among PASC varies greatly in the literature, and the association between PASC and POTS is unclear. Herein, the current literature on POTS and dysautonomia in PASC is reviewed. The results can be used to better understand the overlap and distinction between these pathologies, leading to better understanding of their etiology, treatment, and directions for future research.
METHODSA review of literature on the association of POTS with COVID-19 infection or postinfection was conducted using the Medline (PubMed) database. The search terms used were “COVID-19 and POTS,” “SARS-CoV-2 and POTS,” “COVID-19 and postural orthostatic tachycardic syndrome,” and “SARS-CoV-2 and postural orthostatic tachycardia syndrome,” dated from March 2020 to September 2022. The searches yielded a total of 29 references for potential inclusion based on criteria of English language articles addressing POTS (as in postural orthostatic tachycardia syndrome and not other language uses of POTS) in clinical or seropositive COVID-19 infection or post-COVID-19 infection and case report, clinical study, clinical trial, multicenter study, observational study, or randomized controlled study. Review articles were excluded. Finally, 22 papers were analyzed.
RESULTSCase reports and case series of POTS after acute COVID-19 infection in the early COVID-19 pandemicThe first reported case of POTS early in the pandemic (March 2020) occurred in Orange County, CA, USA and was published by Miglis et al. [6]. A 26-year-old nurse presented with mild cough and an itchy throat, immediately followed by palpitations, fatigue, and mild shortness of breath. For the next month, the orthostatic intolerance (OI) symptoms worsened. The heart rate response to HUTT showed a sustained increase greater than 30 beats/min that persisted for the duration of the tilt during autonomic testing. Based on these findings, she was diagnosed with POTS. Since then, similar cases have been reported, mainly from the USA but a few from European and Asian countries; most patients were young females who presented with fatigue, headache, dizziness, chest pain, and palpitations after COVID-19 infection [6–20]. POTS was diagnosed based on heart rate and blood pressure responses to the HUTT and active standing test in addition to symptoms of chronic fatigue, brain fog, palpitation, OI, and various gastrointestinal (GI) symptoms. POTS symptoms occurred typically a few weeks after the initial upper respiratory infection (URI) symptoms of COVID-19; however, in some cases, POTS symptoms first presented as URI. Most cases included typical symptoms of POTS such as fatigue, headache, dizziness, chest pain, palpitations, nausea/ vomiting, exercise intolerance, and insomnia. Case reports and case series of post-COVID-19 POTS are summarized in Table 1 [6–20].
Observational studies of post-COVID-19 patients focusing on autonomic functionsIn 2021, Buoite Stella et al. [21] published a prospective multidomain observational study of autonomic symptoms and signs in post-COVID-19 patients. They recruited patients who presented with persistent symptoms and/or delayed complications between 4 weeks and 9 months from the onset of COVID-19 infection and were referred to the post-COVID-19 ambulatory service of the University Hospital and Health Services of Trieste, Italy, between February 15 and May 15 2021. Among 180 patients, 13.8% showed OH during the 3-minute active standing test. However, in the short standing test protocol, none of the patients met the criteria for POTS. Subsequently, Wallukat et al. [22] performed a case control study in which functionally active autoantibodies (fAABs) targeting G-protein coupled receptors (GPCR-fAABs) were detected in patients suffering from various long-COVID-19 symptoms. Blood sera were obtained from 31 patients; 29 who were still suffering from post-COVID-19 symptoms after recovery from acute disease and two who were symptom-free. All participants tested positive based on polymerase chain reaction (PCR). Among the patients, seven had POTS. The control group had normal laboratory values. All 31 investigated patients had between two and seven types of GPCR-fAABs. Around the same time, Shouman et al. [23] published a retrospective chart review on post-COVID-19 patients. They included all patients with confirmed history of COVID-19 infection who were referred for autonomic testing at the Mayo Clinic in Rochester, MN or Jacksonville, FL, USA between March 2020 and January 2021 and identified 27 patients who met the inclusion criteria. The results included symptoms after acute infection such as lightheadedness (93%), orthostatic headache (22%), syncope (11%), hyperhidrosis (11%), and burning pain (11%). Orthostatic symptoms without tachycardia were the most common clinical observation. In this population, 22% of patients fulfilled the criteria for POTS, with most patients experiencing orthostatic symptoms showing a normal tilt test. Most recently, Jamal et al. [24] performed a prospective, longitudinal, observational evaluation of patients with PASC symptoms, defined as at least one clinical sequela lasting longer than 4 weeks after acute COVID-19 infection. Patients with abnormal cardiopulmonary functions and prior history of autonomic symptoms were excluded. A total of 24 patients underwent 21-minute HUTT after resting for at least 5 minutes. If the initial HUTT response was normal, the authors administered 0.4-mg sublingual nitroglycerin and observed the response for an additional 15 minutes. Provoked OI (POI) was defined by a heart rate increase of 30 beats/min with no significant changes in blood pressure after administration of sublingual nitroglycerin. In the study, four patients showed POTS and 15 patients showed POI during the HUTT. Only one of 24 patients had normal response on the HUTT, indicating underlying autonomic dysfunction in the majority of post-COVID-19 patients. Observational studies on post-COVID-19 POTS are summarized in Table 2 [21–24].
Case reports of POTS after COVID-19 vaccinationWe found three case reports of POTS after COVID-19 vaccination. Hermel et al. [25] reported a previously healthy 46-year-old female patient who developed POTS symptoms after a single dose of BNT162b2 SARS-CoV-2 vaccine from Pfizer. Her predominant symptoms included fatigue, brain fog, headache, sinus tachycardia, and dizziness. The patient underwent full autonomic testing, which showed normal heart rate variability and Valsalva reactions. However, the HUTT showed significant and sustained elevation of heart rate >40 beats/min, a typical symptom of POTS. Reddy et al. [26] reported a previously healthy 42-year-old male patient who developed POTS symptoms approximately 6 days after receiving the BNT162b2 SARS-CoV-2 vaccine. Extensive medical workup revealed normal heart functions and hormone levels except for sinus tachycardia. In the third case, Park et al. [27] reported a previously healthy 40-year-old male patient who presented with an 8-week history of intermittent headache, palpitation fatigue, and dyspnea, which developed 1 week after receiving the first dose of the Moderna COVID-19 vaccine. The patient underwent full autonomic testing, which showed normal heart rate variability and Valsalva reactions. However, quantitative sudomotor axon reflex test (QSART) showed reduced reflex sweating in the lower extremity. The HUTT showed orthostatic tachycardia with heart rate increasing from 72 to 110 beats/min without significant change in blood pressure. In this case, the patient’s symptoms were nearly resolved at the 5-month follow-up. The case reports of POTS after COVID-19 vaccination are summarized in Table 3 [25–27].
DISCUSSIONSeveral infectious pathogens can trigger chronic sequalae after resolution of acute infections, referred to as post-acute infection syndromes (PAISs). A few PAISs are well-characterized, such as Guillain-Barre syndrome or postpolio syndrome [28]. However, the pathophysiology of PAISs is largely unknown. Therefore, PASC syndrome is very broadly defined as a wide range of any new, returning, or ongoing symptoms at least 4 weeks after acute COVID-19 infection [4]. Despite the uncertainty of the condition, several large cohort studies across the globe have involved longitudinal observation of chronic symptoms beyond several months after acute infection since the pandemic of COVID-19. From these collective efforts, autonomic dysfunction presenting as POTS in PASC has emerged early in the COVID-19 pandemic.
POTS is among the most frequent causes of dysautonomia in the USA and is associated with increased disability and poor quality of life [29]. POTS patients are also frequent visitors to the ED; on average, patients with POTS have nine ED visits prior to diagnosis [30]. POTS disproportionately affects young women, preventing many of them from participating in school or work due to the symptom burden [29]. Although the true prevalence is unknown, POTS is estimated to affect approximately 0.1% to 1% of the US population [31]. In addition, POTS is a clinical syndrome with heterogeneous etiologies. As a final common pathway from multiple different pathophysiological processes, POTS symptoms are primarily caused by impaired vasomotor control of blood volume. For example, damage to sympathetic vasomotor nerves prevents the compensatory increase in systemic vascular resistance to orthostatic changes and/or exercise, causing typical POTS symptoms such as lightheadedness, brain fog, and chronic fatigue. Simultaneously, central, compensatory activation of the sympathetic nervous system causes tachycardia as a response to a reduced cardiac preload; however, the sympathetic activation may also cause other debilitating symptoms of POTS, such as anxiety, insomnia, and GI dysmotility. Although POTS symptoms may appear diverse, they can be clustered into vasomotor and sympathetic symptoms as shown in Fig. 1. In the emergency setting, it is important to understand that POTS patients present with various non-cardiac symptoms, in addition to tachycardia and OI. Furthermore, a study showed that POTS patients visit the ED much less frequently after diagnosis [30]. Therefore, suspecting POTS in the ED is crucial for referring patients to appropriate care in a timely manner.
Notably, typical symptoms of PASC are almost identical to those of POTS, and most can be clustered into vasomotor and sympathetic symptoms. From the cases we reviewed, no specific symptoms were exclusively observed in PASC or POTS, except loss of smell was unique to PASC. Although most patients with loss of smell make a full recovery within one year, a small percentage of patients develop permanent loss of smell [32]. Therefore, hypothetically, PASC syndrome occurs because of autonomic nerve dysfunction, particularly in peripheral sympathetic vasomotor fibers. To support this hypothesis, evidence of sudomotor or small fiber neuropathy in PASC has been increasingly observed in recent studies [33,34]. Sudomotor fibers branch off directly from sympathetic chain ganglia, and impairment of sudomotor fibers can indicate sympathetic nerve dysfunction and subsequent vasomotor dysfunction. Sudomotor function can be evaluated in several ways. As reported by Park et al. [27], the patient with PASC showed reduced QSART, indicating peripheral sympathetic dysfunction. In addition, sudomotor innervation can be quantified using immunohistochemistry staining with PGP9.5 on a cutaneous nerve biopsy [35]. However, direct comparison of sudomotor fiber density between patients with PASC and normal controls has not been performed. Because sympathetic nerve fibers are unmyelinated small fibers, neuropathy can indirectly indicate sympathetic vasomotor dysfunction.
Notably, not all PASC patients who received autonomic testing or active standing test met the criteria for POTS. Autonomic testing showed various cardiovascular responses, including OH, vasovagal syncope, and OI, although these patients presented with similar symptoms and signs. Several explanations are possible. First, diagnosis of POTS was inconsistent among studies. POTS is typically diagnosed based on the HUTT or active standing test. In the active standing test, muscle contraction from gastrocnemius muscles increases cardiac preload, potentially masquerading as orthostatic tachycardia. Plash et al. [36] showed that differences in heart rate during the active standing test are significantly smaller compared with those of the HUTT and suggested that diagnosis of POTS should not be based solely on orthostatic tachycardia. However, consensus is lacking on a specific modality for diagnosing POTS. Second, PASC can possibly affect certain levels of sympathetic vasomotor function, causing specific symptoms, and POTS only occurs when certain areas of sympathetic ganglia are affected. For example, if sympathetic vasomotor function is primarily impaired in splanchnic vasculature, PASC can present predominantly as GI symptoms but not meet the criteria for POTS. Novak et al. [33] recently showed significant reduction of cerebral circulation during the HUTT in PASC patients although their heart rate changes did not meet the criteria for POTS. Therefore, PASC patients with vasomotor symptoms may share the same underlying pathophysiology whether or not they meet the criteria for POTS. Third, symptoms change over time in POTS. In particular, cardiac symptoms, such as palpitation and lightheadedness, usually resolve over time as other symptoms, such as chronic fatigue, brain fog, and GI symptoms, emerge. Considering that the COVID-19 pandemic started approximately 3 years ago, response to the HUTT may change over time in PASC patients in the next several years.
We also found three case reports of POTS after COVID-19 vaccination. Although causality between POTS and vaccines cannot be inferred from these cases, some vaccines are associated with various autoimmune neurological diseases such as Guillain-Barre syndrome or multiple sclerosis [37]. Molecular mimicry between microbial agents and the human host hypothetically plays a role in the development of autoimmunity. In the three cases of postvaccination POTS, the symptoms and signs were almost identical to those of PASC, indicating that COVID-19 vaccines share the same target autoantibody epitope with PASC presenting as immune-mediated POTS. However, this should not discourage patients from receiving vaccines. On the contrary, COVID-19 vaccines can potentially prevent PASC because COVID-19 infection is more likely to cause PASC, and COVID-19 vaccines are proven protective against SARS-CoV-2 virus.
Currently, there are no treatments for post-COVID-19 POTS that target sympathetic vasomotor nerve, although a few clinical trials are testing various immune-modulating agents for postCOVID-19 POTS. The main treatment for post-COVID-19 POTS is volume expansion to increase cardiac preload. Various strategies exist to achieve volume expansion in POTS patients. First, patients are instructed to increase water and sodium intake. The standard amount of water and sodium recommended for POTS patients has not been established; however, in many clinics, patients are recommended to drink 2 to 4 L of water and ingest 3 to 4 g of sodium per day. To achieve volume expansion, consuming water and sodium as fast as possible is also important. Second, certain medications can be used to facilitate volume expansion. Fludrocortisone or α1 agonists, such as midodrine, can improve vasomotor symptoms including brain fog and OI. Stimulants (e.g., methylphenidate) are frequently used for brain fog and fatigue but should be used with caution because they can worsen sympathetic symptoms such as insomnia or palpitations. Third, graded cardiopulmonary training can significantly enhance blood volume over an extended period. However, it is important to understand that patients with POTS have difficulty tolerating exercise due to vasomotor dysregulation as they often develop postexercise flare or postexercise malaise if they overexert themselves. Therefore, slowly advancing their exercise capacity over a long period of time is critical. Last, if POTS is suspected in the ED, an active standing test can be performed to identify orthostatic tachycardia, assuming the patient’s cardiac functions are normal. For acute volume expansion in the ED, a bolus infusion of intravenous normal saline can be delivered to improve the symptoms of POTS. In addition, a short-acting α1 agonist, such as midodrine or fludrocortisone, can be used to optimize volume expansion in conjunction with aggressive hydration.
As the COVID-19 pandemic continues into its 3rd year, PASC is emerging as a global health threat. An increasing need exists for better clinical phenotyping and studying pathophysiology of PASC. However, recognizing certain PASC subtypes, such as POTS, in an emergency setting is important because treatments are available for POTS.
REFERENCES1. Finelli L, Gupta V, Petigara T, Yu K, Bauer KA, Puzniak LA. Mortality among US patients hospitalized with SARS-CoV-2 infection in 2020. JAMA Netw Open 2021; 4:e216556.
2. Nka AD, Ka’e AC, Bouba Y, et al. Global burden of SARS-CoV-2 infection, hospitalization and case fatality rate among COVID-19 vaccinated individuals and its associated factors: a systematic review and meta-analysis protocol. PLoS One 2022; 17:e0272839.
3. Alimohamadi Y, Tola HH, Abbasi-Ghahramanloo A, Janani M, Sepandi M. Case fatality rate of COVID-19: a systematic review and meta-analysis. J Prev Med Hyg 2021; 62:E311-20.
4. Centers for Disease Control and Prevention (CDC). COVID-19: long COVID [Internet]. CDC; 2022 [cited 2022 Dec 1]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html#print.
5. Raj SR, Guzman JC, Harvey P, et al. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol 2020; 36:357-72.
6. Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn DI, Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res 2020; 30:449-51.
7. Kanjwal K, Jamal S, Kichloo A, Grubb BP. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag 2020; 11:4302-4.
8. Umapathi T, Poh MQ, Fan BE, Li KF, George J, Tan JY. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin Auton Res 2020; 30:571-3.
9. Ishibashi Y, Yoneyama K, Tsuchida T, Akashi YJ. Post-COVID-19 postural orthostatic tachycardia syndrome. Intern Med 2021; 60:2345.
10. Ocher RA, Padilla E, Hsu JC, Taub PR. Clinical and laboratory improvement in hyperadrenergic postural orthostatic tachycardia syndrome (POTS) after COVID-19 infection. Case Rep Cardiol 2021; 2021:7809231.
11. O’Sullivan JS, Lyne A, Vaughan CJ. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep 2021; 14:e243585.
12. Johansson M, Stahlberg M, Runold M, et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep 2021; 3:573-80.
13. Petracek LS, Suskauer SJ, Vickers RF, et al. Adolescent and young adult ME/CFS after confirmed or probable COVID-19. Front Med (Lausanne) 2021; 8:668944.
14. Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res 2021; 69:205-11.
15. Buchhorn R, Willaschek C, Baumann C. SARS-CoV-2 infections and the autonomic nervous system. Monatsschr Kinderheilkd 2021; 169:645-8.
17. Schofield JR. Persistent antiphospholipid antibodies, mast cell activation syndrome, postural orthostatic tachycardia syndrome and post-COVID syndrome: 1 year on. Eur J Case Rep Intern Med 2021; 8:002378.
18. Bosco J, Titano R. Severe post-COVID-19 dysautonomia: a case report. BMC Infect Dis 2022; 22:214.
19. Agnihotri SP, Luis CV, Kazamel M. Autonomic neuropathy as post-acute sequela of SARS-CoV-2 infection: a case report. J Neurovirol 2022; 28:158-61.
20. Desai AD, Boursiquot BC, Moore CJ, et al. Autonomic dysfunction post-acute COVID-19 infection. HeartRhythm Case Rep 2022; 8:143-6.
21. Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J Neurol 2022; 269:587-96.
22. Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun 2021; 4:100100.
23. Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 2021; 31:385-94.
24. Jamal SM, Landers DB, Hollenberg SM, et al. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J Am Coll Cardiol 2022; 79:2325-30.
25. Hermel M, Sweeney M, Abud E, et al. COVID-19 vaccination might induce postural orthostatic tachycardia syndrome: a case report. Vaccines (Basel) 2022; 10:991.
26. Reddy S, Reddy S, Arora M. A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus 2021; 13:e14837.
27. Park J, Kim S, Lee J, An JY. A case of transient POTS following COVID-19 vaccine. Acta Neurol Belg 2022; 122:1081-3.
28. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained postacute infection syndromes. Nat Med 2022; 28:911-23.
29. Bourne KM, Chew DS, Stiles LE, et al. Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. J Intern Med 2021; 290:203-12.
30. Shaw BH, Stiles LE, Bourne K, et al. The face of postural tachycardia syndrome: insights from a large cross-sectional online community-based survey. J Intern Med 2019; 286:438-48.
31. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med 2019; 285:352-66.
32. Renaud M, Thibault C, Le Normand F, et al. Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis. JAMA Netw Open 2021; 4:e2115352.
33. Novak P, Mukerji SS, Alabsi HS, et al. Multisystem Involvement in post-acute sequelae of coronavirus disease 19. Ann Neurol 2022; 91:367-79.
34. Novak P, Giannetti MP, Weller E, et al. Network autonomic analysis of post-acute sequelae of COVID-19 and postural tachycardia syndrome. Neurol Sci 2022; 43:6627-38.
35. Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 2004; 127(Pt 7):1606-15.
Fig. 1.Postural orthostatic tachycardia syndrome (POTS) symptom cluster. POTS symptoms can be divided into sympathetic vasomotor dysfunction and sympathetic overcompensation. Because POTS patients experience symptoms of both sympathetic dysfunction and sympathetic hyperfunction, medications should be used with caution. For example, β-blockers can improve tachycardia and anxiety but worsen orthostatic intolerance and fatigue. 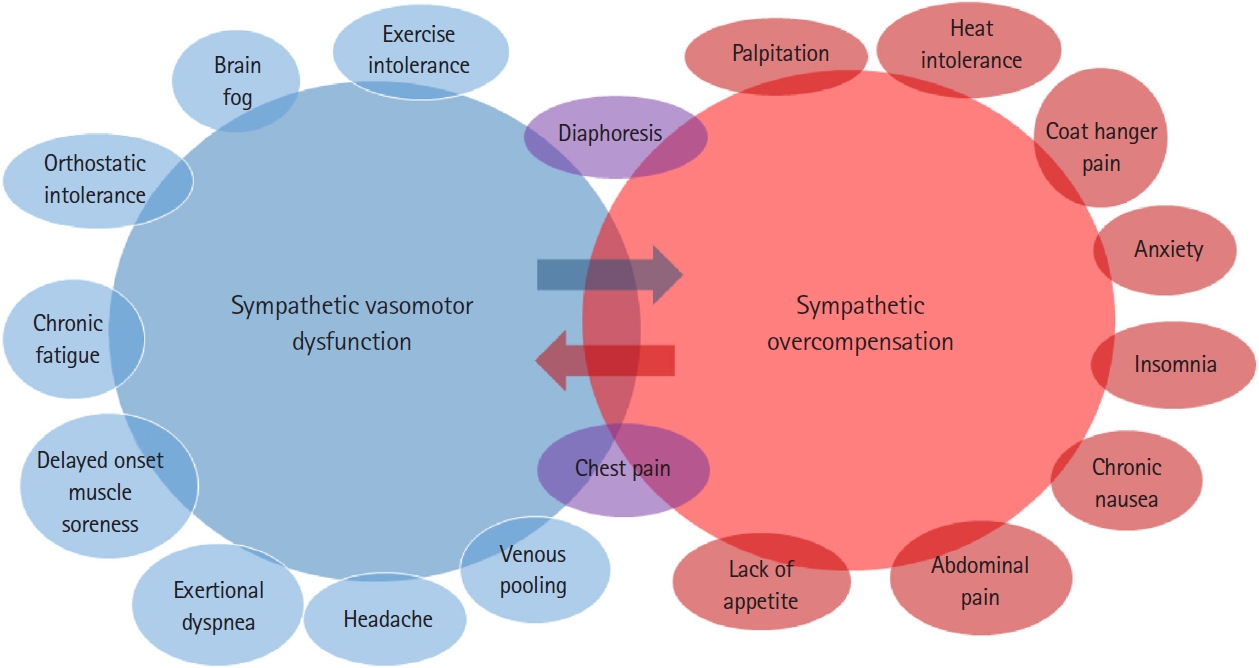
Table 1.Case reports and series of POTS after acute COVID-19 infection
Table 2.Observational studies of autonomic functions in post-COVID-19 patients
fAAb, functionally active autoantibody; GPCR, G-protein coupled receptors; POTS, postural orthostatic tachycardia syndrome; COMPASS-31, Composite Autonomic Symptom Score 31; OH, orthostatic hypotension; OI, orthostatic intolerance; PASC, post-acute sequelae of SARS-CoV-2; HUTT, head-up tilt table test; POI, provoked orthostatic intolerance. Table 3.Case reports of POTS after COVID-19 vaccination
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||