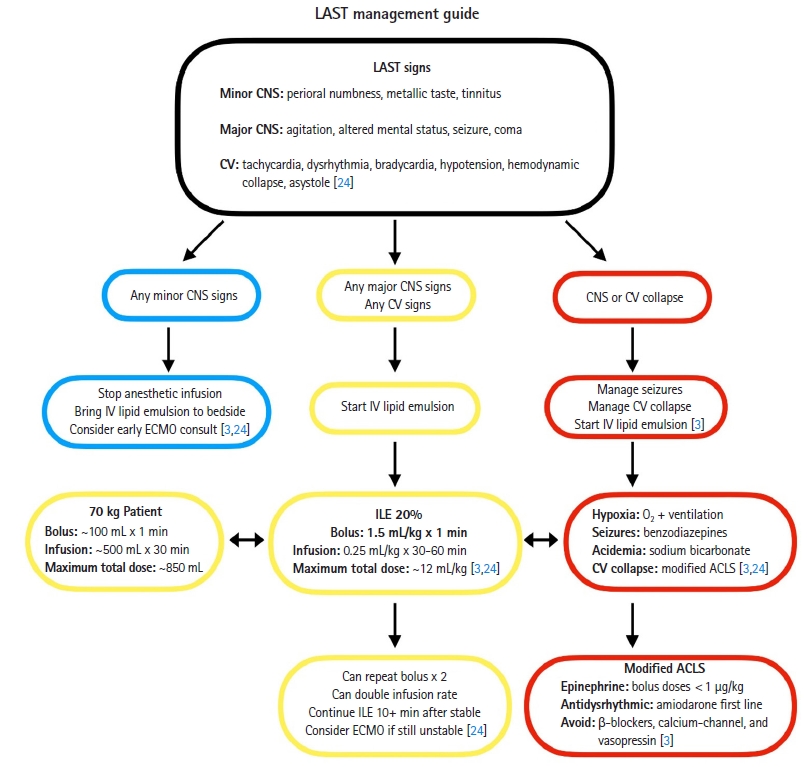
Local anesthetic systemic toxicity (LAST) is a rare but potentially devastating adverse event resulting from the uptake of local anesthetic (LA) in the central nervous system (CNS) and cardiovascular system. Though uncommon, the incidence of LAST is clinically relevant [1]. Owing to its low incidence, underreporting, and the misrecognition of widely varying presentations, the true incidence of LAST is difficult to ascertain [2]. Because of the paucity of data, the American Society of Regional Anesthesia (ASRA) has established a practice advisory rather than a formalized clinical practice guideline [3]. Therefore, any physician who employs LA, including emergency physicians, should be knowledgeable on proper injection techniques, patient risk factors and selection, and reversal guidelines for LAST [3]. In this editorial, the authors discuss the epidemiology, pathophysiology, recognition, and treatment of LAST.
EPIDEMIOLOGY
Given the rarity of the condition, there are no randomized controlled trials evaluating LAST. The current understanding of the incidence of LAST is drawn primarily from large databases but also includes registries, case series, and case reports. Presently, the two largest databases include the Premier Perspective Database [1] and the National Inpatient Sample [2]. However, both of these registries are large, retrospective databases. The Premier Perspective Database only includes information on “billable procedures, diagnoses, and medications,” and thus is subject to misrecognition, treatment, and documentation of LAST [1]. Furthermore, the National Inpatient Sample excluded any relevant information on the block method and LA dosing [2]. Other large registries, such as the Dartmouth registry, demonstrate even lower incidences of LAST but are derived from single-center practices [4]. Thus, while the true incidence of LAST is difficult to determine, perhaps a single numerical risk of this complication after injection with LA is not applicable. First, an ever-growing diversity of regional anesthesia techniques exist. Some peripheral nerve blocks can be performed with only small amounts of anesthetic, while fascial plane blocks, brachial plexus blocks, and paravertebral blocks tend to employ higher volumes and are more proximal to the heart, spine, and CNS [5,6]. Moreover, various types, concentrations, and volumes of LAs are employed in regional anesthesia. While most blocks consist of a single dose of LA, catheter placement provides larger volumes with continuous drug elution. In addition, blocks are performed by physicians from a variety of specialties with varying degrees of expertise. Therefore, an understanding of the relation of types and concentrations of LA, methods of blockade, and patient risk factors is paramount to gauging an individual patient’s risk of experiencing LAST, which varies from patient to patient.
PATHOPHYSIOLOGY
Local anesthetics act at several cellular targets but mainly on voltage-gated sodium channels, and to a lesser extent voltage-gated calcium and potassium channels, within which they inhibit ion transfer and prevent sensory and motor axonal signaling [7]. Bupivacaine additionally uncouples oxidative phosphorylation within mitochondria, halting adenosine triphosphate (ATP) synthesis [8]. While these mechanisms anesthetize peripheral nerves, they cause toxic CNS and cardiovascular system effects. In the CNS, blockade of sodium channels induces either excitation or depression, giving rise to an array of symptoms ranging from excitatory prodromal symptoms (such as paresthesias, metallic taste, or tinnitus) to more drastic symptoms such as altered mental status, seizures, or coma [8]. In the myocardium, inhibition of voltage-gated sodium channels leads to impaired cardiac contractility, conduction, and ultimately coronary ischemia [3]. ATP depletion within myocardial mitochondria further worsens cardiac function [9]. In the vasculature, low concentrations of anesthetic increase systemic vascular resistance, while high concentrations lower it [8]. Therefore, hypertension and tachycardia tend to develop early or in mild toxicity, while bradycardia, dysrhythmias, and reduced ventricular systolic function herald cardiac arrest or more severe toxicity [8]. Electrocardiogram (ECG) abnormalities, such as prolongation of the PR and corrected QT intervals, widening of the QRS, bundle branch blocks, or complete atrioventricular dissociation, may also arise [8].
RECOGNITION AND TREATMENT
LAST is often underrecognized and, like many adverse outcomes, underreported. Recent data suggests that “atypical LAST,” which consists of more subtle signs (cardiovascular dysfunction alone without CNS excitation, or more minor CNS symptoms such as paresthesias and tinnitus), may account for nearly half of all cases [3]. Delayed presentations, occurring greater than 5 minutes after injection but even up to 1 hour, are also becoming increasingly common (or at least better recognized) [3]. Furthermore, LAST is not isolated to regional anesthesia techniques. Recent case series demonstrate that up to 20% of cases arise from simple tissue infiltration with LA [10–12]. Therefore, all patients should be on cardiorespiratory monitoring before receiving a LA and subtle neurologic complaints or signs of cardiac dysfunction (such as tachycardia or hypertension) should alert the physician to halt the procedure and stop administering LA immediately. Patients should be monitored for at least 30 minutes after injection [13]. According to ASRA’s most recent practice advisory on LAST, “[l]ocal anesthetic systemic toxicity is a masquerader, and its detection requires persistent open-minded vigilance” [3].
The mainstay of LAST treatment is intravenous lipid emulsion (ILE), a mixture of triglycerides and egg phospholipid [8]. The lipid droplets contain a strongly negatively charged phospholipid “shell” with a vigorous attractive force for positively charged drugs [14]. Positively charged, fat-soluble LA binds to negatively charged lipid particles [3]. Intralipid emulsion was originally proposed to act as a lipid “sink” that binds free anesthetic in the plasma [15]. However, ILE has since been linked to a number of cellular mechanisms that reverse LAST. Most significant is the role of ILE as a “lipid shuttle”: rather than directly binding and excreting LA, ILE “shuttles” it from areas of high blood flow (heart, CNS) to skeletal muscle and liver, where it is stored and metabolized [16]. This process occurs within minutes of administration of ILE [8]. Bupivacaine, the most lipophilic LA, has a significantly positive charge. In vitro models have demonstrated that bupivacaine binds most tightly and is the most responsive to ILE, while less lipophilic anesthetics such as lidocaine and ropivacaine do not respond as robustly [17]. Thus, the solution lies in the redistribution of LA rather than binding and prompt elimination. By restoring voltage-gated sodium conduction [18] and shortening the half-life of LA [19], ILE reduces mortality [8]. In addition to its role as a lipid shuttle, ILE also exhibits cardiotonic and postconditioning effects. In its cardiotonic effect, ILE increases cardiac contractility, which then increases cardiac output, drug redistribution to skeletal muscle and liver tissue, and thus more timely metabolism [20]. Via its postconditioning effect, ILE increases blood pressure by improving peripheral vascular tone [3], possibly via inhibition of nitric oxide signaling [21] or by modifying adrenergic sensitivity [22]. However, this mechanism is less well-understood. According to ASRA, in cardiac arrest secondary to LAST, oxygenation takes precedence over cardiac support “to prevent the hypoxia, hypercapnia, and acidosis that potentiate LAST and negatively impact resuscitative efforts” [3]. Animal models suggest lower doses of epinephrine are favored (1 μg/kg or less) to avoid impaired oxygenation and increased afterload [23]. All physicians treating LAST should follow the ASRA algorithm, which provides treatment guidelines and clarifies and delineates presentations of LAST (Fig. 1) [3,24]. Of note, ASRA recommends modifying existing Advanced Cardiovascular Life Support algorithm and the evidence supporting this recommendation is not strong.
RISK MITIGATION IN THE EMERGENCY DEPARTMENT
While no single measure can prevent LAST, emergency physicians should be aware of risk factors, how to mitigate them, and when to consider alternate forms of analgesia. In general, the risk of LAST is an aggregate of the physician’s skill and technique, the type and dose of anesthetic used, and the patient’s comorbidities [13]. Concerning technique, ultrasound use has been demonstrated to reduce the incidence of LAST alone by 65% [25], presumably because it enables the physician to directly visualize blood vessels prior to injection. Furthermore, the addition of small amounts of epinephrine can help mitigate LAST, because intravascular injection of even 10 to 15 μg of epinephrine per milliliter of LA administered can induce an increase in heart rate by greater than 10 beats/min [3]. Physicians should always aspirate before injection, even with ultrasound guidance, and should slowly inject incremental aliquots of 5 mL of LA to allow potential systemic absorption to develop before the entire dose is administered. If patients have reduced cardiac output, or in the case of lower extremity blocks in which the site of injection is further away from the heart, physicians should wait up to 15 to 30 seconds between aliquots [13]. All patients should be monitored for at least 30 to 45 minutes after blockade, due to the risk of delayed LAST [26]. Emergency physicians should always use the lowest possible dose and concentration of anesthetic, never exceeding maximum dosing guidelines for lean body weight. While there are multiple dosing guidelines for LAs, the authors employ those in Miller’s Basics of Anesthesia [27]. Physicians should be aware that bupivacaine is the most lipophilic and therefore most toxic LA [3].
Patient factors and comorbidities play a substantial role in the development of LAST. Well-vascularized areas are at greater risk for increased absorption of LA. Therefore, paravertebral, intercostal, and fascial plane blocks impart a higher probability for LAST [13]. Furthermore, such blocks also tend to be performed with larger doses of LA, given both the distance of the target nerves from the site of injection and the number of nerves being targeted with a single block. Patients at extremes of age are at higher risk of LAST due to decreased muscle mass (and thus less tissue to absorb and metabolize LA) [3], as well as having generally lower concentrations of voltage-gated sodium channels in tissues (increasing their susceptibility to the toxic effects of LA) [28]. Chronic cardiac and renal diseases are also crucial risk factors for the development of LAST. Patients with reduced ejection fraction circulate LA more slowly, and thus deposition into muscle and liver tissue is delayed. Patients with coronary artery disease are more susceptible to ischemia [13]. Patients with preexisting dysrhythmias have a lower threshold for developing LA-induced dysrhythmias [13]. Lastly, alpha-1 acid glycoprotein in the plasma binds LA, and thus patients with reduced concentrations of this protein such as infants and pregnant women are also at higher risk for LAST [24].
While LAST is a rare complication of LA administration, it harbors devastating consequences. An individual patient’s risk for LAST is determined by several factors, including age, comorbidities, gestational status, the type and dose of LA, and the block to be performed. Physicians’ skill in regional anesthesia techniques is also important in the prevention of LAST. Therefore, physicians should carefully consider patients deemed to be suitable candidates for regional anesthesia, and they should educate the patient before injection. Emergency physicians should also be vigilant in recognizing LAST early by ensuring that patients are on a cardiorespiratory monitor, adding epinephrine to LA whenever possible, and monitoring for subtle cardiac and neurologic signs of toxicity. Emergency departments in which physicians perform regional anesthesia should always carry ILE, which should be nearby and easily accessible. Emergency physicians who perform regional anesthesia should be familiar with the ASRA checklist for the treatment of LAST or know where and how to conveniently access it. All physicians should report LAST if they suspect it has occurred. Given the rarity of the disease, online registries and databases both serve as an opportunity for physicians to learn about different cases and to document the incidence of LAST [29]. As relative newcomers to the practice of regional anesthesia, emergency physicians should exercise extra caution to perform these techniques properly and to select patients appropriately. Lastly, special consideration should be made for nerve blocks with well-established indications, such as the fascia iliaca compartment block for hip fractures [30] or the erector spinae plane block for rib fractures [31].
CONCLUSION
While the incidence of LAST is rare, this feared complication can be devastating. Emergency physicians should be familiar with the effects of LAs, which patients are at increased risk, and how to mitigate this risk in the emergency department. Lastly, emergency physicians who inject LA should be familiar with the ASRA checklist for the treatment of LAST, and ILE should always be nearby.